In this issue of Blood, Ren et al report the results of a broad genomic and transcriptomic analysis of hepatitis B virus (HBV)–associated diffuse large B-cell lymphomas (DLBCLs) in Chinese patients, providing for the first time a distinctive molecular profile of these tumors.1
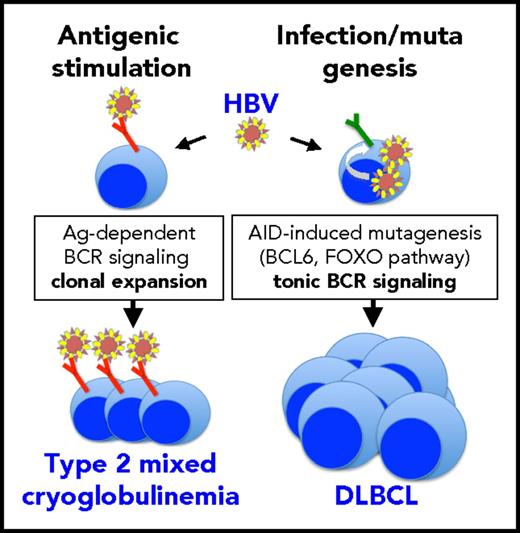
HBV might exploit 2 different mechanisms for causing either indolent (mixed cryoglobulinemia) or highly malignant (DLBCL) B-cell lymphoproliferative disorders. In the case of DLBCL, HBV infection of B cells may enhance overall mutagenesis (possibly through APOBEC enzymes) and alter B-cell–specific signaling pathways (possibly through AID). Among the latter, alterations in the FOXO pathway might result in lymphomagenic tonic B-cell receptor signaling. DLBCL cells do not seem to express stereotyped idiotypes putatively recognizing HBV antigens. Conversely, in mixed cryoglobulinemia, continual antigenic stimulation by HBV may drive the clonal expansion of B cells expressing specific stereotyped idiotypes that regress upon suppression of infection by antiviral therapy. There is no evidence suggesting the possibility of progression from mixed cryoglobulinemia to DLBCL.
HBV might exploit 2 different mechanisms for causing either indolent (mixed cryoglobulinemia) or highly malignant (DLBCL) B-cell lymphoproliferative disorders. In the case of DLBCL, HBV infection of B cells may enhance overall mutagenesis (possibly through APOBEC enzymes) and alter B-cell–specific signaling pathways (possibly through AID). Among the latter, alterations in the FOXO pathway might result in lymphomagenic tonic B-cell receptor signaling. DLBCL cells do not seem to express stereotyped idiotypes putatively recognizing HBV antigens. Conversely, in mixed cryoglobulinemia, continual antigenic stimulation by HBV may drive the clonal expansion of B cells expressing specific stereotyped idiotypes that regress upon suppression of infection by antiviral therapy. There is no evidence suggesting the possibility of progression from mixed cryoglobulinemia to DLBCL.
Among the 275 DLBCL samples characterized by whole-genome sequencing and whole-exome sequencing, 20% were from HBV infection surface antigen–positive (HBsAg+) patients. This high proportion, related to endemic HBV in China, provides a unique opportunity for investigating differences in clinical and mutational spectra between HBV-related and -unrelated DLBCL in the context of a common ethnic background. HBsAg+ DLBCL patients compared with HBsAg– patients had significantly younger age, more aggressive disease, and shorter survival, similar to the findings in another study in Chinese patients.2 Genome-wide analysis revealed an increased total mutation load in HBsAg+ DLBCL possibly resulting from APOBEC enzyme activity and also a distinctive set of mutated genes that might be related to the activity of the B-cell–specific activation-induced cytidine deaminase (AID). BCL6 was the lymphoma-related gene most frequently mutated (79%) in HBsAg+ DLBCL, together with other genes involved in the FOXO signaling pathway (CXCR4, KLF2, and SGK1). The authors speculate that alterations of the FOXO pathway might promote the development of HBsAg+ DLBCL by inducing antigen-independent tonic B-cell receptor signaling, and they suggest BCL6 as an important therapeutic target.
HBV and hepatitis C virus (HCV) share hepatotropism and the capacity to induce B-cell lymphomas.3 In the case of HCV, it is generally agreed that lymphomas develop as a consequence of the protracted stimulation of B cells expressing specific stereotyped idiotypes putatively directed to a viral antigen that has not yet been identified.4 A major argument in favor of this hypothesis is the frequent regression of indolent lymphomas associated with HCV, but not with HCV+ DLBCL, after the clearance of infection with antiviral therapy.5 Ren et al characterized the V(D)J region of immunoglobulin (Ig) heavy chain in 15 HBsAg+ DLBCL samples and were unable to identify recurrent stereotyped idiotypes. This contrasts with another study2 in HBsAg+ DLBCL Chinese patients that identified 2 stereotyped sequences in 4 of 16 patients studied and claimed significant homology of these antibodies to antibodies specific for HBsAg.
On the basis of their results, Ren et al rejected the hypothesis that HBV, like HCV, causes lymphomas by protracted antigenic stimulation of B cells that produce virus-specific antibodies and suggest that infection of B cells by HBV may induce a hyperactive status that leads to enhanced mutagenesis mediated in part by APOBEC and AID. Indeed, these 2 hypotheses might not necessarily be mutually exclusive. In the case of HCV, for which an antigen-driven mechanism of lymphomagenesis is generally accepted, it has also been shown6 that infection of B cells by the virus induces a mutator phenotype that leads to a five- to 10-fold increase in mutation frequency in BCL6, Ig heavy chain, and p53 genes. Conversely, it is also known that some HBV-infected individuals develop type 2 mixed cryoglobulinemia, a monoclonal B-cell lymphoproliferative disorder that can regress after infection is suppressed by antiviral therapy.7 This suggests antigenic pressure as the cause of monoclonal lymphoproliferation because that is the case for HCV-related mixed cryoglobulinemia. HCV causes a large spectrum of lymphoproliferative disorders ranging from the most frequent mixed cryoglobulinemia, which is benign but highly prone to neoplastic evolution, to indolent lymphomas mainly originating from marginal zone B cells, to aggressive DLBCL. This suggests that evolution from benign monoclonal lymphoproliferation to aggressive lymphoma may be driven by continual antigenic stimulation and accumulation of mutations. By contrast, HBV rarely causes mixed cryoglobulinemia, and HBV-related indolent lymphomas are much rarer than HBV-related DLBCL in Chinese patients.8 This large predominance of DLBCL over indolent lymphoproliferative disorders supports a model of HBV-driven mutagenesis directly leading to DLBCL rather than that of stimulation-driven progression from indolent to aggressive forms. HBV-driven mutagenesis might occur by a hit-and-run mechanism, because Ren et al could not detect any HBV DNA integrated into DLBCL cells. Nevertheless, the continual antigenic stimulation model still stands as the most likely explanation for HBV-dependent mixed cryoglobulinemia (see figure).
The large body of data from Ren’s study provides a framework for deciphering the mechanisms by which HBV alters the B-cell genome up to development of DLBCL and, importantly, identifies potential therapeutic targets for these aggressive tumors. Future studies should clarify whether the distinctive molecular signatures found in HBsAg+ DLBCL Chinese patients are also present in patients of Western origin. In addition, the issue of whether virus-specific stereotyped idiotypes may be involved in some HBV-associated monoclonal lymphoproliferative disorders2,7 should be further addressed.
Conflict-of-interest disclosure: The authors declare no competing financial interests.