In this issue of Blood, Statler et al report the incidence and outcomes of patients who were treated on leukemia clinical trials conducted by Southwest Oncology Group (SWOG) from 2005 to 2015, but who were retrospectively deemed ineligible. The primary finding is that patients labeled as ineligible for the included clinical trials have similar outcomes as eligible patients.1
The National Cancer Institute recently introduced initiatives to expand opportunities for adult enrollment into cancer clinical trials, including precision medicine clinical trials and large nationally conducted phase 2 and 3 clinical trials. This is because an abysmal proportion of patients, fewer than 5% of adult patients with cancer, are treated on an investigational protocol.2,3 Restrictive screening requirements have been cited as one barrier to clinical trial enrollment.4,5 Screening a patient for a clinical trial requires diagnosis confirmation, organ function determination, performance status assessment, review of laboratory data, and in some cases, repeat marrow studies. Studies must be “in window” and often need to be repeated. Is such stringent screening warranted?
Statler and colleagues explore that question by studying patients who were treated on SWOG clinical trials but who were retrospectively found to be ineligible. Statler and colleagues identified 247/2361 (10.4%) ineligible patients on 13 phase 2 and 3 SWOG leukemia protocols from 2005 to 2015. Ineligible patients included those who never received treatment on study (78/247, 32%) and those who were both treated on study (169/247, 68%) and included in SWOG analyses but were retrospectively found to be ineligible. Among the 169 treated patients, reasons for ineligibility included missing baseline documentation (60%), laboratory and bone marrow values outside of the protocol-defined time window (25%), and missing documentation about diagnosis (2%). Baseline disease and patient characteristics among eligible and ineligible patients, including Eastern Cooperative Oncology Group performance status, were similar.1
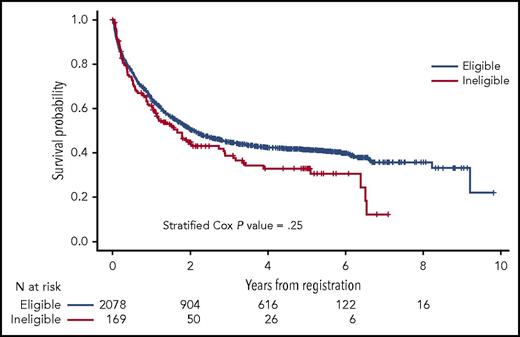
The authors assessed outcomes among eligible and ineligible patients. In 6/8 of evaluable trials, there was no difference in complete remission (CR) rates between eligible and ineligible patients. In the remaining 2 studies, ineligible patients had lower (S0325) and higher (S0703) CR rates compared with eligible patients. Across all studies, there was no difference in overall survival (OS) among eligible and ineligible patients (P = .25) (see figure). This result persisted in a multivariable model that controlled for age, gender, study design, and disease (hazard ratio = 1.12; 95% confidence interval, 0.89-1.40; P = .37). Toxicity was also felt to be equivalent with no difference in grade 5 (fatal) adverse events between cohorts.1
OS of eligible vs ineligible patients. See Figure 1 in the article by Statler et al that begins on page 2782.
OS of eligible vs ineligible patients. See Figure 1 in the article by Statler et al that begins on page 2782.
This study highlights the administrative and organizational barriers to trial enrollment, as ineligibility in most cases was attributable to administrative burdens of documentation or out-of-window laboratory or pathology data. Ineligible patients did not exhibit different survival than eligible patients, which suggests that restrictive criteria, particularly with regard to short study windows, may not affect clinical outcomes. These findings question the culture of the clinical trial screening process. We do not know if baseline assessments were not completed vs were completed but not recorded. If the error is incomplete assessments, is this because of fragmentation of responsibility of research staff, and can the burdens of documentation for research staff be streamlined and simplified? Furthermore, does the process need to liberalize laboratory screening windows and requirement of repeat samples except where specific correlative studies are required? With less restrictive screening criteria, would trial enrollment increase and speed the development of novel therapies?
The Statler et al article underscores one important piece of the puzzle in improving clinical trial enrollment. However, it should be emphasized that this is the tip of the “barriers to clinical trial recruitment” iceberg. We do not know which patients are excluded from screening for clinical trials because of lack of awareness or acceptance, timely referrals, or resources at academic centers to support clinical trial enrollees. Future studies must address barriers at all levels including patient, community provider, and academic provider level.3,6-9 Patients require education about the goals of trials, understanding of risk, and support of logistics of transitioning to an academic medical center.3 Referring providers must have easy access to timely referrals (particularly relevant in aggressive hematologic cancers) and not experience untenable administrative burden and financial repercussions of referring patients outside of one’s practice. At academic centers, resources for efficient screening must be in place, as well as support for patients and their families as they transition to an academic center, often a distance from their referring practice. Finally, as the population of hematologic cancer patients ages, there is an increasing need to better understand comorbidity and then design and enroll older patients with increased comorbidity.10 By addressing organizational, patient, and provider challenges in clinical trial enrollment, interventions may be developed to improve clinical trial recruitment for patients with hematologic cancers and bridge the gap between clinical trial and real-world patients.
Conflict-of-interest disclosure: The author declares no competing financial interests.