Abstract
Disseminated intravascular coagulation (DIC) is a condition characterized by systemic activation of coagulation, potentially leading to thrombotic obstruction of small and midsize vessels, thereby contributing to organ dysfunction. At the same time, ongoing consumption of platelets and coagulation proteins results in thrombocytopenia and low concentrations of clotting factors, which may cause profuse hemorrhagic complications. DIC is always secondary to an underlying condition, such as severe infections, solid or hematologic malignancies, trauma, or obstetric calamities. A reliable diagnosis of DIC can be made through simple scoring algorithms based on readily available routine hemostatic parameters. The cornerstone of supportive treatment of this coagulopathy is management of the underlying condition. Additionally, administration of heparin may be useful, and restoration of physiological anticoagulants has been suggested, but has not been proven successful in improving clinically relevant outcomes so far. In patients with major bleeding or at risk for hemorrhagic complications, administration of platelet concentrates, plasma, or coagulation factor concentrates should be considered.
Introduction
A variety of disorders, including severe sepsis, systemic inflammatory conditions, trauma, and malignant disease, will lead to activation of the coagulation system. In many cases, this coagulation will not result in clinical complications and will not even be identified by routine laboratory tests, but can only be detected when sensitive molecular markers for activation of coagulation pathways are used.1 However, if the activation of coagulation is sufficiently strong, consumption of platelets and coagulation proteins may become visible through prolongation of routine clotting tests and increasing thrombocytopenia. Systemic activation of coagulation in its most extreme form is known as disseminated intravascular coagulation (DIC). DIC is classically characterized by the simultaneous occurrence of widespread vascular clot deposition, compromising an adequate blood supply to various organs, and thereby contributing to organ failure.2-5 Due to ongoing activation of the coagulation system and other factors, such as impaired synthesis and increased degradation of coagulation proteins and protease inhibitors, exhaustion of factors and platelets may occur, potentially resulting in profuse bleeding from various sites. In addition, high levels of fibrin degradation products may affect platelet function and fibrin cross-linking and thereby further contribute to the bleeding tendency.6,7
Extreme bleeding may dominate the clinical picture in patients with DIC; however, this occurs in only a minority of patients.3 The incidence of major bleeding (ie, intracranial, intrathoracic, or intra-abdominal bleeding, or bleeding requiring transfusion) in patients with DIC was 5% to 12% in previous studies.8,9 Patients with DIC and a platelet count of <50 × 109/L have a four- to fivefold higher risk for bleeding as compared with patients with a higher platelet count.10-12
More common is the occurrence of thrombosis in small and midsize vessels contributing to organ failure in patients with DIC, with reported ranges from 10% to 15% in patients with cancer or trauma and up to 40% in patients with sepsis, although these estimates may not be very precise.13,14
A variety of organs in patients with DIC show intravascular fibrin deposition at pathological examination related to the clinical dysfunction of the organs.15 Experimental DIC in animals causes intra- and extravascular fibrin deposition in the kidneys, lungs, liver, and brain, and amelioration of the hemostatic defect improves organ failure and, in some cases, mortality.15,16 In addition, DIC has been shown to be an independent and relatively strong predictor of organ dysfunction and mortality in critically ill patients.9,17 In patients with sepsis and DIC, mortality is almost 2 times higher as compared with patients who do not have DIC.
After a general introduction on the settings in which DIC may occur and a brief overview of current insights into the pathogenesis of DIC, we will use 4 clinical cases to highlight the main clinical dilemmas encountered when managing DIC in clinical practice.
Clinical settings
It should be emphasized that DIC is not a disease in itself, but is always secondary to an underlying condition that causes the activation of coagulation. The disorders most frequently associated with DIC are listed in Table 1.
About 35% of cases of severe sepsis may be complicated by DIC.15,18 Classically, infection with Gram-negative microorganisms has been associated with DIC; however, the incidence of DIC in patients with Gram-positive infections is similar.19 Systemic infections with other microorganisms, including fungi or parasites may lead to DIC as well.19 For example, high parasitemia, primarily of Falciparum malaria, may be associated with DIC and high mortality.20 Factors involved in the development of DIC complicating infections are microbial membrane constituents, such as lipopolysaccharide or lipoteichoic acid, or exotoxins (eg, Staphylococcal α-toxin), evoking a strong immune response and release of cytokines.
Both hematological malignancies and solid tumors may be complicated by DIC due to the expression of procoagulant factors by tumor cells.21 The incidence of DIC in cancer is not precisely known and may depend on the diagnostic criteria used; however, some series, in particular in patients with metastasized adenocarcinoma or lymphoproliferative disease, report an incidence of up to 20% in consecutive cases.22 The risk of thrombosis is greater than bleeding, and in severe cases, thromboembolism in conjunction with bleeding can be seen.23
Severe trauma is another clinical condition commonly associated with DIC.2,24 Systemic cytokine patterns in patients with severe trauma have been shown to be virtually identical to those of septic patients.25 In addition, release of tissue material (such as tissue thromboplastin, in particular in patients with head trauma) into the circulation and endothelial disruption may contribute to the systemic activation of coagulation. It may be difficult to differentiate DIC from the coagulopathy due to massive blood loss and the dilutional coagulopathy as a result of massive transfusion or infusion of large volumes of crystalloids that may occur in the first hours after major trauma.26
In obstetric calamities, such as placental abruption and amniotic fluid emboli, acute and fulminant DIC may occur.27,28 The degree of placental separation in patients with abruptio placentae correlates with the extent of DIC, suggesting that leakage of tissue factor from the placental system into the maternal circulation is responsible for the occurrence of DIC.
For other underlying conditions (Table 1), DIC is a relatively infrequent complication. In most situations, the severity of the associated systemic inflammatory response in combination with specific circumstances, such as concomitant infections, will determine whether severe systemic coagulation activation will occur.
Pathogenetic pathways
The most important mechanisms leading to the pathological derangement of coagulation in DIC have been clarified. The initiation and propagation of procoagulant pathways with simultaneous impairment of natural anticoagulant systems and suppression of endogenous fibrinolysis as a result of systemic inflammatory activation are leading to platelet activation and fibrin deposition.15,29 Important mediators that regulate these processes are cytokines, such as interleukin-1 (IL-1) and IL-6 and tumor necrosis factor-α (TNF-α). In addition, recent studies point to a prominent role of intravascular webs (neutrophil extracellular traps) consisting of denatured DNA from damaged cells and entangling neutrophils, platelets, fibrin, and cationic proteins, such as histones, in the development of thrombus deposition.30
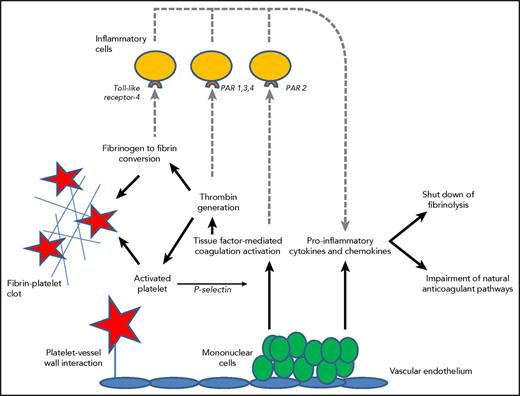
Thrombin generation in DIC is initiated through the tissue factor/factor VII(a) pathway that activates downstream coagulation factors.31 Tissue factor may be expressed by activated monocytes, but also by vascular endothelial cells or cancer cells. Besides inflammation causing procoagulant effects, the activation of coagulation also modulates inflammation (Figure 1).
Mononuclear cells express tissue factor resulting in thrombin generation and subsequent fibrinogen to fibrin conversion, which, in combination with increased platelet vessel wall interaction and activation of platelets, contribute to microvascular clot formation. P-selectin released from activated platelets further upregulates tissue factor expression. Binding of fibrin to Toll-like receptor 4 and activated coagulation proteases to specific protease activated receptors (PARs) on inflammatory cells (dotted lines) modulates inflammation through the additional release of proinflammatory cytokines. Cytokines also play a key role in the suppression of endogenous fibrinolysis and impairment of physiological anticoagulant pathways, such as the APC pathway.
Mononuclear cells express tissue factor resulting in thrombin generation and subsequent fibrinogen to fibrin conversion, which, in combination with increased platelet vessel wall interaction and activation of platelets, contribute to microvascular clot formation. P-selectin released from activated platelets further upregulates tissue factor expression. Binding of fibrin to Toll-like receptor 4 and activated coagulation proteases to specific protease activated receptors (PARs) on inflammatory cells (dotted lines) modulates inflammation through the additional release of proinflammatory cytokines. Cytokines also play a key role in the suppression of endogenous fibrinolysis and impairment of physiological anticoagulant pathways, such as the APC pathway.
In DIC, all physiological anticoagulant pathways are functionally impaired.15 Firstly, a marked imbalance of TFPI function in relation to the increased tissue factor–dependent activation of coagulation has been described.32 In addition, significant impairment of the protein C system may further compromise adequate regulation of thrombin generation. This is caused by a cytokine-mediated downregulation of thrombomodulin expression on endothelial cells in combination with decreased synthesis of protein C and a fall in the concentration of the free fraction of protein S (the essential cofactor of protein C), together resulting in reduced activation of protein C.33 Lastly, plasma levels of antithrombin, the most prominent inhibitor of thrombin and factor Xa, are significantly reduced in DIC due to a combination of consumption, degradation by elastase from activated neutrophils, and impaired synthesis.34 In addition, the endogenous fibrinolytic system is largely shut off as a result of a sustained rise in the plasma level of plasminogen activator inhibitor-1 (PAI-1), the principal inhibitor of plasminogen activation and plasmin generation.2
Patient 1: diagnosis of DIC in a 68-year-old critically ill man
A 68-year-old man was admitted to the intensive care unit because of respiratory failure 3 days after a hemicolectomy. His blood pressure was 100/60 mm Hg, heart rate was 120 beats per minute (regular), respiratory rate was 28 breaths per minute, and temperature was 38.1°C. Arterial blood gas analysis showed a Pao2 of 8.4 kPa (63 mmHg) and oxygen saturation of 84% while on 5 L supplemental oxygen. Laboratory analysis showed a hemoglobin concentration of 6.8 mmol/L (11.0 g/dL), a leukocyte count of 9.2 × 109/L with 7.7 × 109/L neutrophils, a remarkable left shift and some schistocytes in the blood smear, a creatinine concentration of 212 μmol/L (2.4 mg/dL), a bilirubin concentration of 18 μmol/L (1.2 mg/dL), a lactic acid concentration of 3.3 mmol/L (30 mg/dL), and a bicarbonate concentration of 18 mmol/L. The patient was intubated and ventilated, achieving an oxygen saturation of 98%, and hemodynamically stabilized with crystalloids and IV administration of dopamine. He was further treated with broad spectrum antibiotics and continues subcutaneous heparin prophylaxis.
Routine coagulation tests showed a platelet count of 98 × 109/L (152 × 109/L the day before), a prothrombin time (PT) of 17 seconds (normal, <12 seconds), which equals an international normalized ratio (INR) of 1.4, an activated partial thromboplastin time () of 43 seconds (normal, <28 seconds), a D-dimer concentration of 7.5 μg/mL (normal, <0.5 μg/mL), and a fibrinogen concentration of 3.5 g/L (normal, 1-3 g/L).
Comments about patient 1
This critically ill patient with multiple organ failure and a clinical suspicion of sepsis showed clear signs of a coagulopathy, characterized by a low platelet count, prolonged global coagulation tests, and increased D-dimer. These laboratory abnormalities may be compatible with DIC, however, some differential diagnostic considerations need to be taken into account.
Sepsis per se is clearly associated with thrombocytopenia, and the severity of sepsis correlates with the reduction in platelet count.35 The principal factors that contribute to thrombocytopenia in patients with sepsis are impaired platelet production, increased consumption or destruction, or sequestration of platelets in the spleen or along the endothelial surface. In addition, in a considerable number of patients with sepsis, marked hemophagocytosis may occur. This pathologic process consists of active phagocytosis of megakaryocytes and other hematopoietic cells by monocytes and macrophages, hypothetically due to stimulation with high levels of macrophage colony-stimulating factor (M-CSF).36 Platelet activation, consumption, and destruction may also occur at the vascular surface as a result of extensive endothelial cell–platelet interaction in sepsis, which may differentially occur in vascular beds of various organs.37 These mechanisms alone, however, do not explain a prolongation of coagulation times.
The presence of thrombocytopenia and schistocytes in the blood film may point in the direction of a thrombotic microangiopathy, such as thrombotic thrombocytopenic purpura or hemolytic-uremic syndrome. However, these syndromes are typically accompanied by normal clotting times and normal or only slightly elevated D-dimer.38 Schistocytes may also be seen in patients with DIC as a result of enhanced platelet-vessel wall interaction and the formation of microvascular thrombotic obstruction, causing mechanical damage to erythrocytes. Interestingly, patients with severe sepsis and DIC may have reduced ADAMTS-13 levels (presumably due to consumption as a result of the large amount of von Willebrand factor multimers released from perturbed endothelial cells), causing some overlap between DIC and thrombotic microangiopathy.39 Raised von Willebrand factor levels and the related reduced ADAMTS 13 levels are associated with worse outcomes in severe DIC.40
Another differential diagnostic consideration for the thrombocytopenia in this case may be heparin-induced thrombocytopenia (HIT).41 It is likely that our patient was treated with subcutaneous heparin for some days as perioperative thrombosis prophylaxis. Patients with HIT may present with arterial and venous thrombosis, which may explain a high D-dimer result. However, HIT is not associated with abnormal global coagulation times.
Taken together, DIC is the most probable explanation for the coagulopathy in this patient. Patients with DIC have a low or rapidly decreasing platelet count, prolonged global coagulation tests, low plasma levels of coagulation factors and inhibitors, and increased markers of fibrin formation and/or degradation, such as D-dimer or fibrin degradation products.42 Coagulation proteins with a marked acute phase behavior, such as factor VIII or fibrinogen, are usually not decreased or may even increase. One of the often-advocated laboratory tests for the diagnosis of DIC, fibrinogen, is therefore not a very good marker for DIC, except in very severe cases, although sequential measurements can give some insight. Dynamic changes in coagulation factors and platelets may add important information. A significant drop in platelet count (as illustrated in this case), a lengthening duration of clotting assays, or an increase in fibrin split products, even still within the normal range, can indicate an early stage of developing DIC.9
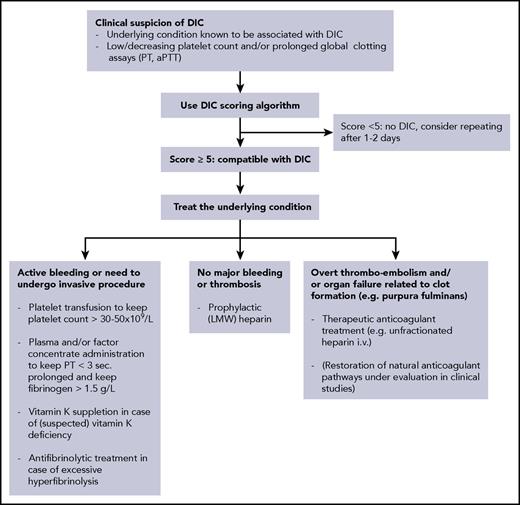
There is no single laboratory test with sufficient accuracy for the diagnosis of DIC. For the diagnosis of DIC, a simple scoring system (Table 2, Figure 2) has been developed by the International Society on Thrombosis and Hemostasis.43,44 The score can be calculated based on routinely available laboratory tests (ie, platelet count, prothrombin time, a fibrin-related marker [usually D-dimer], and fibrinogen). Prospective studies have shown that the sensitivity of the DIC score is 93%, and the specificity is 98%.17,45 The severity of DIC according to this scoring system is a strong predictor for mortality in sepsis.46 Similar scoring systems and diagnostic guidance have been developed and extensively evaluated in Japan, Italy, and the United Kingdom.47-49 The major difference between the international and Japanese scoring systems seems to be a slightly higher sensitivity of the Japanese algorithm, although this may be due to different patient populations, because Japanese series typically include relatively large numbers of patients with hematological malignancies.
Flowchart for the diagnostic and therapeutic management of DIC. LMW, low molecular weight.
Flowchart for the diagnostic and therapeutic management of DIC. LMW, low molecular weight.
In our patient, the platelet count (1 point), prolongation of the PT (1 point), and strongly increased D-dimer (3 points) led to a score of 5 points, compatible with a diagnosis of DIC.
Patient 2: a 63-year-old woman with DIC or coagulopathy related to liver disease?
A 63-year-old woman, with long-standing alcohol abuse, presented with decompensated liver cirrhosis. At physical examination, the most prominent signs were hepatic encephalopathy, jaundice, splenomegaly, and ascites. Laboratory test results showed a hemoglobin concentration of 7.0 mmol/L (11.3 g/dL), a leukocyte count of 7.9.2 × 109/L, a platelet count of 88 × 109/L (1 week before admission, 84 × 109/L), a bilirubin concentration of 84 μmol/L (1.2 mg/dL), and an albumin concentration of 28 g/L. Coagulation tests reveal a PT of 17 seconds (normal, <12 seconds), an aPTT of 52 seconds (normal, <28 seconds), a D-dimer concentration of 1.0 μg/mL (normal, <0.5 μg/mL), and a fibrinogen concentration of 2.1 g/L (normal, 1-3 g/L). The question is whether these coagulation abnormalities are due to liver failure-related coagulopathy or DIC, secondary to an infection.
Comments on patient 2
In patients with severe hepatic failure, several changes in coagulation may occur. More than 75% of patients with cirrhosis present with a platelet count of <150 × 109/L, and in > 10% of patients, the platelet count is <75 × 109/L, most importantly caused by sequestration of platelets in the enlarged spleen, reduced levels of thrombopoietin, and consumption.50,51 In addition, plasma levels of almost all coagulation factors (except factor VIII and von Willebrand factor) are low, as the liver is the most important site of coagulation protein synthesis. The combination of thrombocytopenia and low levels of coagulation factors was traditionally interpreted as a hypocoagulable state and associated with a high risk of bleeding. However, recent insights point to a rebalanced hemostatic system in patients with chronic liver failure, as low levels of natural coagulation inhibitors may balance low levels of coagulation factors and the reduced platelet count may be offset by high levels of von Willebrand factor.52,53
The differential diagnosis between the coagulopathy of liver disease and DIC is challenging as many laboratory abnormalities point in the same direction. Even more complex situations may occur when the coagulopathy of liver disease is complicated by DIC, as patients with severe liver disease may present with infectious complications (such as bacterial peritonitis) or leakage of endotoxin from the intestinal compartment that may elicit DIC. However, in most cases, the coagulopathy of liver disease can eventually be distinguished from the presence of DIC.54 Helpful clues may be that, in contrast to patients with DIC, in severe liver disease, the (low) platelet count is usually stable, and fibrin degradation products (such as D-dimer) are only mildly elevated due to the simultaneous presence of fibrinolytic activation and impairment in this condition.55-57 In addition, clinical signs, such as the presence of splenomegaly and ascites, may indicate that liver disease rather than DIC is the cause of the coagulopathy. In the case presented, the DIC score was 4 (suggestive of no DIC), and, in combination with the clinical signs and symptoms, a diagnosis of coagulopathy due to severe liver failure was made.
Patient 3: supportive treatment in a 63-year-old man with sepsis and DIC
A 63-year-old man presented with severe cholangiosepsis due to an obstructive stone in the common bile duct. He was in shock, respiratory insufficient, and developed acute renal failure. Blood cultures were positive for Klebsiella pneumoniae.
Coagulation analysis showed a platelet count of 48 × 109/L, a PT of 19 seconds (normal, <12 seconds), which equals an INR of 1.6, an aPTT of 39 seconds (normal, <28 seconds), a D-dimer concentration of 5.5 μg/mL (normal, <0.5 μg/mL), and a fibrinogen concentration of 2.8 g/L (normal, 1-3 g/L). Based on these findings, the DIC score was 6 and a diagnosis of DIC was established.
Awaiting endoscopic retrograde cholangiopancreatography and restoration of bile duct patency, the patient was treated with vasopressors, intubation, and mechanical ventilation, and antibiotic treatment was started. The question is what would be the most appropriate (supportive) treatment of the coagulopathy.
Comments about patient 3
The keystone in the management of DIC is adequate treatment of the underlying disorder. If the condition causing the DIC is properly dealt with (in the example of the case with bile duct drainage and antibiotics), the coagulopathy will spontaneously resolve. However, in some situations, adjunctive supportive treatment aimed at the coagulation system will be required, because the coagulopathy may proceed for a while even after adequate treatment of the underlying condition has been initiated (Figure 2).42,58,59
Low levels of platelets and coagulation factors may increase the risk of bleeding, in particular in postoperative patients or those planned to undergo an invasive intervention. However, plasma or platelet substitution therapy should not be instituted on the basis of laboratory results alone; it is indicated only in patients with active hemorrhage and in those requiring an invasive procedure or who are otherwise at risk for bleeding complications.48,58 The presumed efficacy of treatment with plasma, fibrinogen, cryoprecipitate, or platelets is not underpinned by randomized controlled trials, but appears to be rational therapy in bleeding patients or in patients at risk for hemorrhage with a significant deficiency of these hemostatic factors. The use of large volumes of plasma may be required to restore normal levels of coagulation factors. Coagulation factor concentrates, such as prothrombin complex concentrate, may overcome this impediment, but these agents may lack important factors (eg, factor V). Previously, the use of prothrombin complex concentrates was thought to aggravate the coagulopathy in DIC due to small traces of activated factors in the concentrate. It is, however, not very likely that this is still the case for the currently available concentrates. Specific deficiencies in coagulation factors, such as fibrinogen, may be corrected by administration of purified coagulation factor concentrates. Vitamin K must be remembered as a useful non–blood product alternative to correcting vitamin K–dependent factors and will work within 4 to 6 hours after a dose.
Experimental studies have shown that heparin can at least partly inhibit the activation of coagulation in DIC.60 However, a clinically relevant effect of heparin in patients with DIC has never been unequivocally demonstrated in controlled clinical trials, although indirect evidence is accumulating that heparin might be of benefit.61,62 In addition, there are several studies showing that all critically ill patients need adequate prophylaxis for venous thromboembolism, usually with (low–molecular-weight) heparin.63 Therapeutic doses of heparin are indicated in patients with clinically overt thromboembolism and may be considered in cases of extensive thrombotic manifestations, such as in purpura fulminans or acral ischemia.
Restoration of the levels of physiological anticoagulants in DIC may be a rational approach and has been extensively evaluated.34 Based on successful preclinical studies, the use of anti-thrombin concentrates has been evaluated in randomized controlled trials in patients with severe sepsis. All trials have shown some beneficial effect in terms of improvement of laboratory parameters or even improvement in organ function. An international multicenter, randomized controlled trial with an anti-thrombin concentrate, however, did not demonstrate a significant reduction in mortality of septic patients.64 Interestingly, post-hoc subgroup analyses of this study indicated some benefit in patients who did not receive concomitant heparin and in those with the most severe coagulopathy.65 Recent propensity-adjusted retrospective data from Japan demonstrated a significant advantage of anti-thrombin–treated patients with severe infection and DIC.66,67 However, these observations require prospective validation.
Adjunctive therapy with activated protein C (APC) has also been widely studied. A phase III trial of APC concentrate in patients with sepsis was prematurely terminated because of a significant reduction of mortality in these patients.68 Notably, patients with overt DIC benefited most from this treatment.46 However, after a series of negative trials in specific populations of patients with severe sepsis, meta-analyses of published studies concluded that the basis for treatment with APC was lacking.69 In addition, there was ambiguity regarding the hemorrhagic risk of APC in patients with severe sepsis. The last large placebo-controlled trial in patients with severe sepsis and septic shock was prematurely stopped due to the absence of any significant advantage of APC.70 Subsequently, the manufacturer of APC has withdrawn the product from the market.
A promising intervention that is currently being evaluated is recombinant soluble thrombomodulin. Preclinical experimental sepsis studies have demonstrated that soluble thrombomodulin is capable of ameliorating the derangement of coagulation and may improve organ dysfunction.71,72 Recombinant soluble thrombomodulin was evaluated in a phase II/III clinical study in 750 patients with sepsis and DIC.73 Twenty-eight-day mortality was 17.8% in the thrombomodulin group and 21.6% in the placebo group. The encouraging results with soluble thrombomodulin were confirmed by retrospective data in large series of Japanese patients and are being prospectively investigated in a currently ongoing multicenter phase III trial.74
Patient 4: a 48-year-old woman with acute promyelocytic leukemia and severe DIC
A 48-year-old female presented with severe persisting epistaxis. She also reported gingival bleeding and widespread ecchymosis for 1 week and unusually severe menorrhagia. Laboratory analysis showed a hemoglobin concentration of 5.5 mmol/L (8.9 g/dL), a leukocyte count of 21.7 × 109/L, with 90% (19.5 × 109/L) promyelocytes, 4% (0.9 × 109/L) myelocytes, 3% (0.7 × 109/L) metamyelocytes, and 2% (0.4 × 109/L) neutrophils, and a platelet count of 56 × 109/L. The bone marrow contained few blast-like cells with scant granules and 72% promyelocytes with Auer rods. Immunophenotyping showed expression of CD9, CD13, and CD33 and was negative for CD34. Fluorescence in situ hybridization of the bone marrow aspirate revealed a t(15;17) translocation and the PML-RARα fusion gene was confirmed by reverse transcriptase polymerase chain reaction, all compatible with a diagnosis of acute promyelocytic leukemia (M3 subtype of acute myeloid leukemia [AML-M3], according to the French–American–British classification).
Coagulation analysis revealed a PT of 20 seconds (normal, <12 seconds), which equals an INR of 1.7, an aPTT of 59 seconds (normal, <28 seconds), a D-dimer concentration of 10 μg/mL (normal, <0.5 μg/mL), and a fibrinogen concentration of 0.8 g/L (normal, 1-3 g/L). The plasma levels of plasminogen and α2-antiplasmin were 68% (normal, 80%-120%) and 43% (normal, 80%-100%), respectively. Taken together, these results indicate the presence of DIC in combination with marked hyperfibrinolysis.
Comments about patient 4
There is ample evidence for a procoagulant state in virtually all patients with advanced malignant disease. This may eventually lead to venous thromboembolism or evolve into DIC.75 DIC can be diagnosed in up to 15% of consecutive patients presenting with acute leukemia, in particular acute lymphoblastic leukemia, and some reports indicate that the incidence of DIC in acute leukemia patients might further increase during remission induction with chemotherapy.76
A special manifestation of DIC may occur in some patients with advanced adenocarcinoma, but more frequently in patients with acute promyelocytic leukemia (AML-M3) and monocytic leukemias (eg, the M5 subunit of AML) at the time of diagnosis.77 These patients may present with a severe and sometimes life-threatening bleeding tendency. The hemorrhagic tendency is a consequence of DIC with consumption of coagulation factors and platelets in combination with striking hyperfibrinolysis. Hyperfibrinolysis can be diagnosed by low levels of plasminogen and α2-antiplasmin, low levels of fibrinogen (due to plasmin-mediated fibrinogenolysis), and excessively high levels of fibrin degradation products (eg, D-dimer).78 Further analysis may reveal remarkably low levels of factor VIII and factor V (which are both degraded by plasmin), which is in contrast to more conventional types of DIC, where plasma levels of factor VIII are usually relatively preserved.
The coagulopathy associated with acute promyelocytic leukemia is caused by the expression of tissue factor and cancer procoagulant on leukemic cells in combination with increased expression of annexin II, causing excessive plasminogen activation and fibrinolysis.79,80 Release of nonspecific proteases from granules in the leukemia cells may further contribute to the degradation of both fibrin and fibrinogen.
Management of patients who present with acute promyelocytic leukemia and DIC consists of supportive treatment with platelet transfusion (aiming at a platelet count of >30-50 × 109/L), fresh frozen plasma, and fibrinogen concentrate (guided by the fibrinogen concentration in the patient’s plasma) and should be maintained throughout remission induction and disappearance of the coagulopathy.81 In addition, invasive procedures, such as biopsies or IV line placement, should be avoided as much as possible. In view of the high risk of bleeding and the lack of evidence from clinical studies, the use of heparin is not advocated. Before the introduction of all-trans retinoic acid and arsenic trioxide in the therapy of acute promyelocytic leukemia, adjunctive treatment with fibrinolysis inhibitors (such as tranexamic acid) was shown to be beneficial in small clinical trials.82 However, with these modern treatment modalities, the coagulopathy quickly subsides and inhibition of fibrinolysis is usually not necessary anymore and may even be harmful in view of the prothrombotic features of retinoic acid.83
Final considerations
Although the understanding of DIC and its underlying pathogenetic pathways has increased in recent decades, some important questions regarding the proper management of this coagulopathy remain. Current therapeutic interventions are mostly supportive and only partly effective, at best resulting in an amelioration of the derangement of coagulation or more rapid resolution of DIC; however, they have not been proven to result in an improvement of clinically relevant outcomes, such as organ failure or mortality. Because there is increasing circumstantial evidence that heparin may be beneficial in patients with DIC, a randomized controlled trial of heparin in this situation is urgently wanted.
Better supportive treatment of DIC may also benefit from advances in early patient identification and risk stratification. Hypothetically, assays specifically aimed at the assessment of endothelial cell perturbation in combination with early stage systemic coagulopathy would be helpful to recognize high-risk patients and would facilitate early (and thereby potentially more efficacious) intervention. In addition, genetic variation may play a role in the individual propensity to develop DIC and the severity of the coagulopathy.84 Gene mutations and polymorphisms have been demonstrated to affect hemostatic changes in DIC. Septic mice with heterozygous protein C deficiency due to a 1-allele targeted disruption of the protein C gene presented with more severe DIC and associated inflammatory response.85 The presence of factor V Leiden heterozygosity has been associated with the incidence and outcome of DIC in patients with sepsis.86 In addition, the functional 4G/5G polymorphism in the PAI-1 gene affected plasma levels of PAI-1 and was related to clinical outcome of meningococcal sepsis and DIC.87 A better understanding of the influence of genomic variation in the host response leading to DIC could be helpful in identifying patients that are more susceptible to severe coagulopathy and eventually tailoring treatment to the most vulnerable patients.
Authorship
Contribution: M.L. and M.S. designed the outline of the paper, reviewed the literature, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marcel Levi, Department of Medicine, University College London Hospitals NHS Trust, 250 Euston Rd, London NW1 2PG, United Kingdom; e-mail: marcel.levi@nhs.net.