In this issue of Blood, Rogers et al demonstrate that 3 novel agents approved for chronic lymphocytic leukemia (CLL), ibrutinib, venetoclax, and obinutuzumab, can be safely combined at standard doses to create an active, chemotherapy-free triplet regimen for relapsed or refractory disease.1
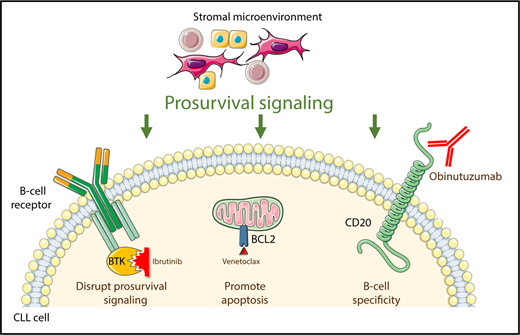
Ibrutinib, venetoclax, and obinutuzumab independently target 3 pathways critical for the survival of neoplastic B cells in CLL.
Ibrutinib, venetoclax, and obinutuzumab independently target 3 pathways critical for the survival of neoplastic B cells in CLL.
Cytotoxic chemotherapy in combination with an anti-CD20 antibody (known as chemoimmunotherapy) has been a widely used treatment for patients with CLL. Each chemotherapeutic agent was initially developed as a single drug. Subsequently, the pioneers of modern CLL therapy found that chemotherapy drugs with differing mechanisms of action, such as the alkylating agent cyclophosphamide and the nucleoside analog fludarabine, could be safely given together and had superior efficacy when given in combination rather than individually.2 The addition of the type I anti-CD20 monoclonal antibody rituximab led to the birth of the fludarabine-cyclophosphamide-rituximab (FCR) regimen, the first CLL therapy to demonstrate an overall survival benefit in a randomized trial.3
The CLL field now stands at a similar juncture. Ibrutinib (an inhibitor of Bruton’s tyrosine kinase [BTK]), venetoclax (an inhibitor of the antiapoptotic protein BCL-2), and obinutuzumab (a type II anti-CD20 monoclonal antibody) have complementary mechanisms of action and toxicity profiles (see figure). Moreover, unlike in the era when combination cytotoxic chemotherapy was developed, there is compelling preclinical evidence that the combination of ibrutinib and venetoclax could be highly active,4 and early results of clinical trials of this doublet and of either agent with obinutuzumab seem promising.5-7 However, until now, the safety and efficacy of a triplet combination was unknown. Rogers et al have provided the first data to begin to address these questions with early results from a phase 1 trial of ibrutinib, venetoclax, and obinutuzumab.
Putting these 3 highly active agents together raises concerns regarding the potential for toxicities of tumor lysis syndrome (TLS) and neutropenia. To mitigate the risk of TLS, Rogers et al took the sensible approach of sequentially introducing the 3 agents, starting with obinutuzumab monotherapy for 1 cycle, adding ibrutinib during the second cycle, and then finally introducing venetoclax in cycle 3. This approach successfully debulked tumor burden, thereby lessening the risk for TLS; no cases of clinical or laboratory TLS were seen in their study. Starting with obinutuzumab did lead to infusion reactions in 83% of patients, and one wonders whether starting the ibrutinib either before the obinutuzumab or at the same time might have mitigated this toxicity while still effectively debulking the patients’ tumors, as was recently suggested in an ongoing study.7 Another safety concern when combining obinutuzumab and venetoclax is neutropenia, but fortunately the rate of grade ≥3 neutropenia in the Rogers et al study was 33% (with no episodes of febrile neutropenia), which is comparable to the 37% rate reported in a recent comprehensive safety analysis of venetoclax monotherapy.8 Thus, the authors appropriately concluded that venetoclax could be safely administered at the full 400 mg daily dose approved for monotherapy, thereby allowing for maximal dose intensity of the triplet combination.
This 12-patient phase 1 experience with relatively short follow-up (median, 24.4 months) cannot definitively answer questions about the efficacy of this triplet compared with a doublet or monotherapy; however, the high rate of undetectable minimal residual disease in the bone marrow after 14 cycles of therapy (5 [83%] of 6 patients at the highest dose level, including 3 patients with a concomitant complete response) certainly merits further investigation. These tantalizing early results suggest the possibility that the efficacy of this triplet therapy may be more than the sum of its parts. But with highly active doublet combinations such as ibrutinib-venetoclax and venetoclax-rituximab also in the discussion,5,9 it will ultimately take randomized data in a large number of patients to demonstrate superiority of a triplet regimen.
Such studies are now being planned, but there are several considerations for their design that are worthy of consideration. First, as previously mentioned, starting with the BTK inhibitor before or concurrently with the obinutuzumab will likely decrease the rate of infusion reactions.7 Second, next-generation BTK inhibitors such as acalabrutinib may have fewer off-target adverse effects than ibrutinib, an especially important consideration in a triplet combination regimen, in which toxicities from all 3 agents may occur. We recently launched one such study, the phase 2 AVO (NCT03580928; Acalabrutinib, Venetoclax, and Obinutuzumab for Initial Therapy of CLL) trial. Regimens that combine all 3 mechanisms of action carry the theoretical risk that few unused drug classes remain for the treatment of relapsed disease; however, the characteristics of relapsed disease after triplet therapy are currently unknown. It is possible that patients who achieve remission with time-limited triplet therapy can be retreated with the same drugs at time of relapse and respond again. Alternatively, other active agents such as phosphoinositide-3-kinase (PI3K) inhibitors could be used. A similar concern was raised about FCR a decade ago, and yet it was found that putting the three best drugs of that era together in the first-line treatment setting had curative potential in a subset of CLL patients with low-risk disease.10 Although an analogous relationship between triplet novel agent therapy and combination chemoimmunotherapy remains to be proven, this parallel is within the realm of possibility.
If the results from this important early study by Rogers et al are confirmed in larger numbers of patients, this type of approach may herald the dawn of a new era in CLL therapy, in which a fixed-duration, chemotherapy-free triplet can safely provide durable disease control in a diverse array of CLL patients, including those with high-risk disease.
Conflict-of-interest disclosure: M.S.D. served as a consultant or advisory board member for Genentech, Janssen Oncology, Pharmacyclics, TG Therapeutics, AbbVie, Merck, AstraZeneca, Verastem, Gilead Sciences, and Syros Pharmaceuticals; and received research funding from Genentech, Pharmacyclics, TG Therapeutics, Verastem, Bristol-Myers Squibb, Surface Oncology, and MEI Pharma. B.L.L. declares no competing financial interests.