In this issue of Blood, Swimm et al report that the tryptophan metabolite indole that is produced by the intestinal microbiome limits graft-versus-host disease (GVHD). In mouse models of GVHD, they found that indoles act via type I interferons (IFNs) to limit chemotherapy- and radiotherapy-induced damage to the intestinal epithelium.1
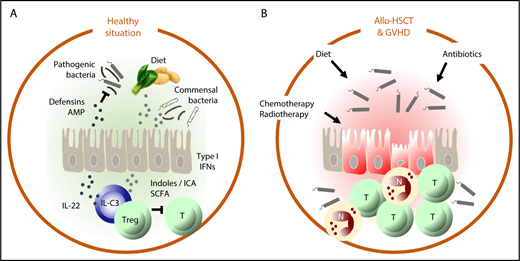
In the healthy situation (A), intestinal homeostasis is maintained by a delicate balance between factors derived from the diet and metabolized by commensal bacteria, such as indoles and short-chain fatty acids (SCFAs) that suppress autoreactive T cells and stimulate epithelial barrier function (directly via upregulation of type 1 IFN and via IL-22) and production of defensins and antimicrobial peptides (AMPs) that suppress outgrowth of pathogenic bacteria. In allo-HSCT (B), chemotherapy and radiotherapy damage the intestinal epithelium and the microbiome directly and indirectly by negatively affecting dietary intake. Neutropenia necessitates the use of antibiotics, which also damages the microbiome. Together, these factors disrupt the homeostatic balance of the intestine, which leads to inflammation, the influx of alloreactive T cells (T) and neutrophils (N), mucositis, bacterial translocation, and GVHD.
In the healthy situation (A), intestinal homeostasis is maintained by a delicate balance between factors derived from the diet and metabolized by commensal bacteria, such as indoles and short-chain fatty acids (SCFAs) that suppress autoreactive T cells and stimulate epithelial barrier function (directly via upregulation of type 1 IFN and via IL-22) and production of defensins and antimicrobial peptides (AMPs) that suppress outgrowth of pathogenic bacteria. In allo-HSCT (B), chemotherapy and radiotherapy damage the intestinal epithelium and the microbiome directly and indirectly by negatively affecting dietary intake. Neutropenia necessitates the use of antibiotics, which also damages the microbiome. Together, these factors disrupt the homeostatic balance of the intestine, which leads to inflammation, the influx of alloreactive T cells (T) and neutrophils (N), mucositis, bacterial translocation, and GVHD.
The relationship between transplantation hematologists and their patients’ gut microbiota has had its ups and downs but has never been as good as in the past years. Taking advantage of novel technologies that no longer depend on culturing to determine the composition of the gut microbiome, Jenq, Taur, and colleagues demonstrated that allogeneic hematopoietic stem cell transplantation (allo-HSCT) is associated with a loss of intestinal microbial diversity that precedes the development of GVHD and has a poor prognosis.2,3 Subsequent studies showed that when certain bacterial families such as the Ruminococcaceae and the Lachnospiracaea were maintained, the risk to develop GVHD was significantly reduced. Indeed, gut epithelium protective properties have been ascribed to bacteria of the class Clostridiales (to which the Ruminococcaceae and the Lachnospiracaea belong). Via production of the short-chain fatty acid butyrate, these bacteria activate regulatory T cells. Moreover, butyrate protects intestinal epithelial cells against apoptosis and enhances epithelial cell junctions, thereby maintaining epithelial barrier function. In experimental GVHD, supplementation of butyrate mitigated GVHD.4 Thus, although gut bacteria may still cause harm when they translocate an inflamed and damaged intestinal epithelium, the immune modulating and epithelial homeostasis promoting properties of the intestinal microbiome are increasingly acknowledged and embraced (see figure).
An important question to be answered is whether immunological and epithelial gut homeostasis is maintained by individual bacterial species or by the diversity of the community. Swimm et al may have added a piece to the puzzle, by demonstrating that indoles, either produced in the intestine by Escherichia coli or added as a compound (the indole derivative indole-3-carboxaldehyde [ICA]) mitigate GVHD in a dose-dependent manner. In a series of comprehensive experiments, they demonstrated that indoles induce type I IFN genes in epithelial cells, whereas in IFN-I receptor knockout mice that received sublethal irradiation therapy (without HSCT), the gut epithelium protective effect of indoles was abrogated. Indoles are metabolites from tryptophan, an essential amino acid that is metabolized by tryptophanase-expressing commensal bacteria. Swimm et al used E coli as an indole-producing commensal in their experiments (and a mutant that lacks the tryptophanase gene). E coli belongs to the family Enterobacteriaceae; other gut commensals that produce indoles are, for example, Enterococcus faecalis (Enterococcaceae) and Clostridium sporogenes (Clostridiaceae). The observation of Swim et al that bacteria-produced indoles and exogenously administered indole derivates mitigate GVHD suggests that the source of indoles and indole derivates is of less importance and that other indole-producing bacteria may have similar effects. In other words, as long as the microbiome as a community provides indoles to protect the gut epithelium against chemotherapy- or radiotherapy-induced damage, the exact composition of the microbiome may be of minor importance.
Indoles have been shown to enhance tight junctions between epithelial cells and enforce the epithelial barrier in the intestine.5 In addition to this direct effect on the gut epithelium, Swimm et al report that indoles induced tolerance (not anergy) of splenic T cells. To what extent T-cell tolerization contributed to the GVHD mitigating effect of indoles remains unclear. Although tolerized splenic T cells in indole-treated mice could well have systemic effects, hepatic GVHD was not affected by indoles. Moreover, indoles did not significantly reduce the influx of alloreactive donor T cells to the intestine or liver (only to the lung), whereas proinflammatory cytokines and chemokines were significantly reduced in the gut of indole-treated mice. Thus, although allogeneic T-cell reactivity was significantly reduced in indole- or ICA-treated mice, it remains to be determined whether this directly affected GVHD severity. Innate lymphoid cells that can be activated by indoles via their Ahr-receptor to produce interleukin-22 (IL-22) did not seem to play a significant role in alleviating GVHD in this model.1
Swimm et al are the first to show a GVHD mitigating effect by bacteria-derived indoles. These data add to the growing amount of evidence that metabolites of commensal bacteria may be of importance to maintain epithelial barrier homeostasis in the context of allo-HSCT and GVHD. Although historically the focus in the laboratory and clinic has been on T-cell alloreactivity, the interest in other pathophysiologic mechanisms of GVHD is growing, and rightfully so, because clinically relevant progress in human GVHD prevention and treatment has not been made in the past decade. As a consequence of the interest in the beneficial effects of commensal bacteria, clinical trials have been initiated in which the impact of modulation of diet (to maintain adequate supplies of essential amino acids like tryptophan), reduction of antibiotic use (to reduce damage to the microbiome), metabolite supplementation (eg, butyrate), and microbiome modulation via fecal microbiota transplantation (via capsules, nasoduodenal tube, or enema) are studied.6 Although preclinical data are promising and ICA can be easily bought via eBay, it is of the utmost importance that we “don’t try this at home” and instead study these type of interventions in the context of clinical trials so that we can learn and adapt.
Conflict-of-interest disclosure: The author declares no competing financial interests.