In this issue of Blood, Hrusak et al show the superiority of acute lymphoblastic leukemia (ALL) type therapy in most cases of ambiguous lineage acute lymphoblastic leukemia (ALAL) in childhood.1 ALL cells commonly express either B-cell antigens (∼75% of cases) or T-cell antigens (20% of cases). In ∼4% of cases, the leukemic cells coexpress B and myeloid antigens, or, more rarely, T plus myeloid antigens, or, extremely rarely, are trilineage positive. In a few cases, no lymphoid lineage-specific antigens are expressed. The cells appear on fluorescence-activated cell sorter staining as a single population in 75% of cases or as bilineal.
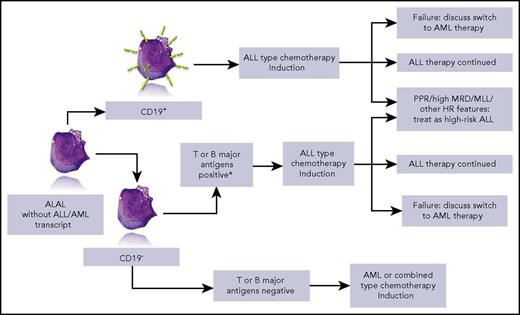
Simplified overview of the strategy that can be drawn from the data of Hrusak et al. HR, high risk; MLL, mixed-lineage leukemia; MRD, minimal residual disease; PPR, prednisone poor responder. *iCD3 and CD7+ or ≥2 of CD10, ICD79a, or iCD22+. Lymphoblast picture by courtesy of Isabelle Sudaka, Hematology Laboratory, CHU de Nice, France.
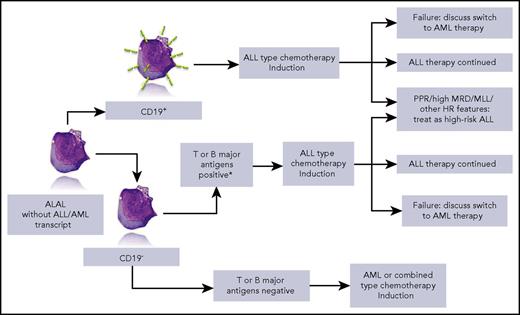
Simplified overview of the strategy that can be drawn from the data of Hrusak et al. HR, high risk; MLL, mixed-lineage leukemia; MRD, minimal residual disease; PPR, prednisone poor responder. *iCD3 and CD7+ or ≥2 of CD10, ICD79a, or iCD22+. Lymphoblast picture by courtesy of Isabelle Sudaka, Hematology Laboratory, CHU de Nice, France.
When first described in children in 1984 by Pui et al, ALAL was named mixed-phenotype acute leukemia2 and was more precisely defined by the European Group for the Immunological Characterization of Leukemias in 1995.3 Biphenotypic acute leukemia was defined through a scoring system based on lineage-specific antigen expression. In 2008, the World Health Organization classification introduced a simpler staging based on fewer surface antigens.4 Although the clinical identification of ALAL is not now a matter of debate, the same cannot be said of the question of optimal therapy for these children.5 Reasons for this situation were the lack of precise recommendations from childhood ALL trials, with ALAL sometimes a reason for trial exclusion, and the lack of data on a sufficient number of patients.
This large retrospective series included patients treated from 2002 to 2015 and excluded patients with acute myeloid leukemia (AML)– or ALL-specific transcripts and BCR-ABL+ patients. The global outcome of ALAL was worse than that of common B-cell ALL, but better than AML. However, striking differences emerge when analyzing event-free survival and survival according to the type of therapy. Five-year event-free survival reached 80% in patients treated with ALL protocols, but was <40% in patients who received AML-type therapies. The outcome of patients treated with combined lymphoid and myeloid-directed therapies was in between. When focusing on patients with CD19+ ALAL, the difference was even more striking in favor of ALL-type therapy. Further conclusions can be drawn from this tremendous collaborative work: when available, minimal residual disease provides prognostic information in this subset of patients, and postinduction therapy should be adapted according to known prognostic factors, including prednisone response and rearrangement of the MLL/KTM2A gene (see figure). The impact of more recently identified genomic factors such as IKZF1 has not been studied in this retrospective series. Allogeneic stem cell transplantation was clearly not mandatory in ALAL, but did help more chemoresistant patients. Treatment options for resistant patients must be approached with caution. For example, in bilineal ALAL, new salvage therapies such as blinatumomab or CD19-CAR-T might be inefficient on the CD19− cell component, thus supporting multivalent peptibody use.6
Finally, based on the results of the study, the authors propose a treatment algorithm that they recommend be tested in a prospective trial. This proposal sounds attractive and, given the paucity of ALAL patients, a common international trial based on the template in this paper would be most desirable.
Conflict-of-interest disclosure: The author declares no competing financial interests.