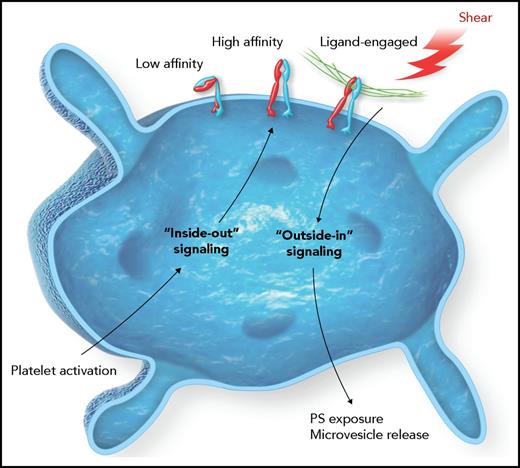
In this issue of Blood, Pang et al define a shear-dependent pathway stimulating integrin outside-in signaling and facilitating platelet procoagulant properties, including membrane phosphatidylserine exposure, microvesicle release, and intravascular fibrin formation.1
The platelet integrin αIIbβ3 resides in a low-affinity state that transitions to a high-affinity state for ligand binding upon platelet activation. This transition is mediated by inside-out signaling. Once bound by a ligand, such as fibrinogen, outside-in pathways are triggered to support more platelet changes, such as spreading and clot retraction. The work by Pang et al demonstrates outside-in signaling is facilitated by shear forces on the integrin mediated by an intracellular G protein that ultimately increases the procoagulant potential of the platelet. PS, phosphatidylserine.
The platelet integrin αIIbβ3 resides in a low-affinity state that transitions to a high-affinity state for ligand binding upon platelet activation. This transition is mediated by inside-out signaling. Once bound by a ligand, such as fibrinogen, outside-in pathways are triggered to support more platelet changes, such as spreading and clot retraction. The work by Pang et al demonstrates outside-in signaling is facilitated by shear forces on the integrin mediated by an intracellular G protein that ultimately increases the procoagulant potential of the platelet. PS, phosphatidylserine.
Normal platelet function is highly dependent upon receiving and processing information via complex signaling pathways. Like most cells, the platelet’s ability to respond is dependent upon receptors in the plasma membrane that connect the platelet’s external environment to internal platelet circuitry. It is within the cytoplasmic milieu that the signal is processed and leads to dynamic changes in platelet structure and function. A key membrane receptor in supporting the platelet’s role in hemostasis and thrombosis is the platelet integrin receptor αIIbβ3. The initial event for αIIbβ3’s engagement is the transition from a low-affinity binding receptor, for ligands such as fibrinogen, to a high-affinity binding receptor. The affinity transition occurs by activation of other platelet receptors and an “inside-out” signaling pathway impacting αIIbβ3 structure via interactions at the cytoplasmic tail of the integrin (see figure). However, integrin signaling is bidirectional such that once ligand binding occurs, receptor clustering follows and “outside-in” signaling leads to even more dramatic changes in platelet behavior.2
The outside-in signaling pathways are both dynamic and complex.3 For example, the extracellular environment in the blood, as measured by flow and shear force, is constantly changing in response to injury and a growing thrombus. In addition, there are spatiotemporal dynamics within the platelet due to overlapping and competing signaling proteins. Mechanisms that elevate intracellular calcium, in the absence of shear, can elicit the procoagulant platelet phenotype. Thus, the challenges to elucidating relevant outside-in pathways are numerous yet extremely important to understand platelet pathophysiology. Nevertheless, there are some generalities for outside-in signaling outcomes, such as platelet spreading and clot retraction.
The current work of Pang et al uses elegant in vitro and in vivo analyses to gain insights into outside-in signaling that are impacted by extracellular shear on the integrin receptor (see figure). Specifically, results document the effect of shear on αIIbβ3 that leads to an increased exposure of procoagulant phosphatidylserine on the platelet surface and the release of platelet microvesicles. Mechanistically, these outcomes are linked to the heterotrimeric guanine nucleotide-binding (G protein) subunit Gα13. The data are acquired using several different approaches, including mutant αIIbβ3 subunits, mutant Gα13 subunits, pharmacologic targeting of Gα13, in vivo analysis of thrombus formation, and in vitro coagulation assays. Thus, the conclusions are based on a thorough and robust testing of the defined pathway.
The relevance of shear to platelet function is not a novel concept. Platelets contain another well-characterized receptor that responds to shear: the glycoprotein Ib-IX complex. In the case of glycoprotein Ib-IX, shear alone can elicit ligand (von Willebrand factor) binding and the release of microvesicles.4 Where the work of Pang et al expands our understanding is a demonstration that shear forces alone are not sufficient to elicit αIIbβ3-dependent procoagulant platelet properties. Agonist stimulation and the β3 subunit are both required to fully engage the Gα13, Src, and Rac-1 dependent signaling pathway.
The authors present compelling data with the pharmacologic inhibition of the Gα13-dependent signaling pathway and the resulting impact on platelet function in vivo. First, the results establish that it is possible to separate inside-out pathways leading to ligand binding from the later consequences of outside-in signaling leading to platelet procoagulant properties (see figure). Inhibition of the Gα13 pathway significantly reduces fibrin deposition within a growing platelet thrombus and effectively destabilizes the thrombus. The authors conclude that selective targeting of integrin outside-in signaling represents a novel antithrombotic strategy that has the potential of inhibiting occlusive thrombus growth with a limited impact on normal hemostasis, the holy grail in the development of improved antithrombotic therapies.5
In considering the inhibition of Gα13 signaling pathways, it is worth remembering that heterotrimeric G proteins and their downstream targets are physiologically relevant well beyond platelet-mediated thrombosis. Indeed, G proteins are widely involved in cell differentiation, proliferation, and motility.6 In the case of the platelet, and specifically Gα13, the G protein is being used to facilitate structural changes leading to a more procoagulant platelet. However, the same G proteins are known to harbor mutations associated with breast cancer.7 Analyzing big datasets has identified inactivating mutations in the gene encoding Gα13 (GNA13) with links to Burkitt lymphoma and diffuse large B-cell lymphoma.8,9 Thus, the existing data suggest that mutations in this pathway contribute to cellular transformations and metastatic potential, possibly behaving as tumor suppressors in some cell types.10 Pharmacologic inhibition of the Gα13/RhoA axis as an antithrombotic strategy will require careful consideration beyond the immediate impact on thrombosis.
Finally, the current work further illustrates the complex spatiotemporal aspects of the platelet’s role in thrombosis. Going forward, the challenge will be to exploit the complexity for the design of a new generation of antithrombotics with limited side effects, such as excess bleeding. While specific targeting of Gα13 might have unexpected consequences as discussed above, it nevertheless identifies the potential to retain some platelet behavior, such as ligand binding, while reducing the platelet’s role in the intravascular coagulation response. The authors are commended for an important and insightful contribution based on solid experimental data.
Conflict-of-interest disclosure: The author declares no competing financial interests.