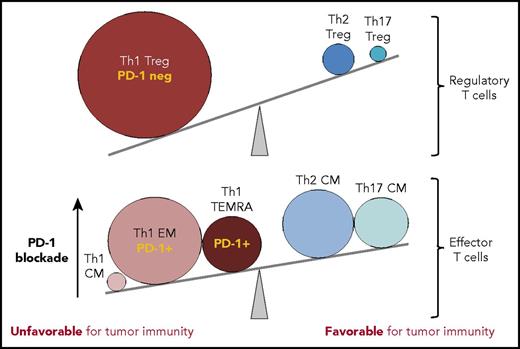
In this issue of Blood, Cader et al dissect the T-cell subsets within the tumor microenvironment of Hodgkin lymphoma, revealing an overabundance of CD4+ regulatory T cell (Treg) and exhausted T helper 1 (Th1) effector cells within the Reed-Sternberg cell's dysfunctional immunologic milieu.1
T-cell subsets within the Hodgkin lymphoma tumor microenvironment tip the balance away from tumor immunity. In comparison to reactive lymph nodes and tonsils, T cells infiltrating cHL tumors have marked expansions in T-cell subsets that can suppress tumor-specific killing (Treg cells, PD-1 low/negative; >10-fold expanded) and EM CD4+ T cells with PD-1 intermediate/high expression (Th1 EM, Th1 TEMRA; >fivefold expanded), rendering them sensitive to the suppressive effects of the high levels of PD-L1 expressed by HRS cells. The effectiveness of PD-1 blockade in cHL may rely on the release of these effector CD4+ T-cell populations from PD-L1-mediated suppression, achieving tumor control presumably via class II MHC-mediated cytotoxicity. CM, central memory. The figure has been adapted from Figure 7C in the article by Cader et al that begins on page 825.
T-cell subsets within the Hodgkin lymphoma tumor microenvironment tip the balance away from tumor immunity. In comparison to reactive lymph nodes and tonsils, T cells infiltrating cHL tumors have marked expansions in T-cell subsets that can suppress tumor-specific killing (Treg cells, PD-1 low/negative; >10-fold expanded) and EM CD4+ T cells with PD-1 intermediate/high expression (Th1 EM, Th1 TEMRA; >fivefold expanded), rendering them sensitive to the suppressive effects of the high levels of PD-L1 expressed by HRS cells. The effectiveness of PD-1 blockade in cHL may rely on the release of these effector CD4+ T-cell populations from PD-L1-mediated suppression, achieving tumor control presumably via class II MHC-mediated cytotoxicity. CM, central memory. The figure has been adapted from Figure 7C in the article by Cader et al that begins on page 825.
The discovery that classical Hodgkin lymphoma (cHL) is extraordinarily responsive to treatment with PD-1 blocking antibodies has begged the question of the effector mechanism(s) behind this singular intervention.2,3 The unique tumor microenvironment of cHL comprises rare malignant Hodgkin Reed-Sternberg (HRS) cells within an extensive inflammatory and immune cell infiltrate, in which the malignant cells evade immune destruction by multiple mechanisms.4 HRS cells almost invariably exhibit increased copy number alterations in the 9p24.1 locus that encodes the programmed death 1 (PD-1) receptor ligands PD-L1 and PD-L2, which engage the PD-1 “checkpoint” receptor on T cells, inhibiting their antitumor immune functions.5
Nevertheless, in cHL, the precise mechanism for PD-1–mediated immune evasion has proved to be more complex than simply “releasing the brakes” on PD-1-expressing CD8+ T cells as it is in some other cancers, because HRS cells usually lack the β2-microglobulin and class I major histocompatibility complex (MHC) expression required for direct cytotoxicity by CD8+ T cells.6 Rather, recent studies point to CD4+ T cells having a critical role in cHL tumor immunity. These observations include that HRS cell expression of β2-microglobulin and class I MHC fails to correlate with outcomes in relapsed/refractory cHL treated with PD-1 blockade,7 the retained high-level expression of MHC class II expression on HRS cells,7 and the topological arrangement of PD-1+, CD4+ T cells interacting with PD-L1+ HRS cells in situ.8
However, the precise phenotype(s) of T cells infiltrating cHL and their roles as effectors have been incompletely characterized. To catalog the breadth of tumor-infiltrating lymphocytes in cHL, Cader and colleagues used the powerful technique of time-of-flight mass cytometry (CyTOF), which permits simultaneous detection of dozens of cell markers using antibodies conjugated to heavy metal ions.9 Their panel of 39 antibody reagents was able to quantitate, at the single-cell level, HRS cells and associated inflammatory and immune cells, including CD4+ and CD8+ T-cell subsets, B cells, natural killer cells, and macrophages. Cellular infiltrates from 7 primary cHLs were compared with 10 reactive lymph nodes or tonsils.
In contrast to the normal lymphoid tissues, cHL had markedly expanded populations of Th1-polarized Treg cells and more differentiated CD4+ Th1-polarized effector cell populations, including “effector memory” (Th1 EM; CCR7− CD45RO+) and terminally differentiated effector memory cells (TEMRA; CCR7− CD45RO− CD45RA+). Although direct T-cell function is not demonstrated in this study, it is rather inferred by the precise surface immunophenotypes identified with the CyTOF analysis. CD4+ T-effector memory (TEM) cells, and in particular the subset that reexpresses CD45RA after antigenic stimulation (CD4 TEMRA), are implicated in protective immunity against viruses and tumors.10 Furthermore, the TEMRA population can be highly enriched for CD4 cytotoxic T lymphocytes, which can exhibit granzyme B-mediated tumor cytotoxicity in a class II MHC-restricted manner.10 These latter CD4+ TEM and TEMRA cells express significant amounts of PD-1, suggesting that they play an important effector role during PD-1 blockade therapy (see figure), during which the inhibitory effects of PD-1 ligation are lifted, potentially tipping the balance back in favor of tumor immunity.
Thus, these new data help to further illuminate specific perturbations in the cHL immunologic “neighborhood,” and how immunotherapy for this disease might be further improved. For instance, the demonstrated pathologic expansion of Th1-polarized Treg cells may suggest that inhibition or depletion of Treg cells might further shift the balance in favor of tumor immunity in cHL. Additional phenotypic or functional in vitro studies of cHL T-cell–HRS cell interactions may also identify other targetable pathways for boosting the effectiveness of immunotherapy against this lymphoma.
Conflict-of-interest disclosure: J.T. received research funding from Bristol-Myers Squibb, Kite, and ImmunGene.