Key Points
Absence of oncogene-specific T cells leads to increased B-cell lymphoma incidence in a new mouse model.
Premalignant lymphoma-initiating B cells are not eradicated by the immune system, retaining the risk of lymphoma development.
Abstract
To date, little is known about the interaction between (pre-)malignant B cells and T cells. We generated transgenic mice that allow B cell-specific induction of the oncogene SV40 large T-antigen (TAg) to analyze the role of oncogene-specific T cells during sporadic B-cell lymphoma development. Constitutive TAg expression in CD19-Cre × LoxP-Tag mice resulted in TAg-tolerant CD8+ T cells and development of B-cell lymphomas. In contrast, CD19-CreERT2 × LoxP-Tag mice retained TAg-competent CD8+ T cells at time of oncogene induction and TAg expression in few B cells of adult mice resulted in exceptionally rare lymphoma formation late in life. Increased lymphoma incidence in the absence of TAg-specific T cells suggested T cell-mediated inhibition of lymphoma progression. However, TAg-initiated B cells were not eliminated by T cells and detected long term. Our results demonstrate a failure of the immune system to eradicate lymphoma-initiating B cells, retaining the risk of lymphoma development.
Introduction
The graft-versus-leukemia effect, recently surpassed by the success of T-cell-based therapies with CD19-specific chimeric antigen receptors, demonstrated the enormous potential of T cells in fighting B-cell malignancies.1 However, little is known about the mechanisms by which (pre)malignant B cells and T cells interact during the long-lasting process from premalignant lymphoma-initiating B cells to lymphoma formation in the endogenous host. The increased lymphoma incidence in immune-suppressed patients is often used to propose immunosurveillance of lymphomas, without differentiating between spontaneous and virus-induced cancer formation2 against which by no doubt a protective T-cell response is in place.3
B-cell lymphomas differ from the majority of solid tumors in that the malignant cells themselves are derived from immune cells. B cells express major histocompatibility complex (MHC) class II and costimulatory molecules and are more easily accessible for T cells. However, ample evidence exists that resting B cells are tolerogenic for naive T cells, via mechanisms of anergy and sensitization to antigen-induced cell death.4-10 Activated B cells, on the other hand, have been described by different studies to either induce4,9,11-13 or not induce tolerance,5,7,14 fail in T-cell priming7,15 or activate naive T cells.16-19 Thymic B cells have even been shown to mediate central tolerance of CD4+ T cells.20,21 Furthermore, persistence and amount of antigen were shown to influence the balance between tolerance and immunity.22-25
How these diverse properties of B cells influence lymphoma development remains not fully understood. To investigate the development and pathology of B-cell neoplasms as well as therapeutic strategies, virus-induced mouse models26,27 and transgenic (eg, c-myc, LMP-1/2) or gene-deficient (eg, p53, Blimp-1) mice prone to develop B-cell malignancies have been generated in the past.28-34 Some of these studies demonstrated control of lymphoma development by natural killer cells, T cells, or T-cell mediators.33-39 These models have given important insights into disease development but have 3 drawbacks: (1) they do not recapitulate the sporadic nature of nonviral B-cell lymphomas, (2) no antigen is known for which the mice are not yet tolerant at birth, and/or (3) no T-cell epitope is known to study specific T-cell responses.
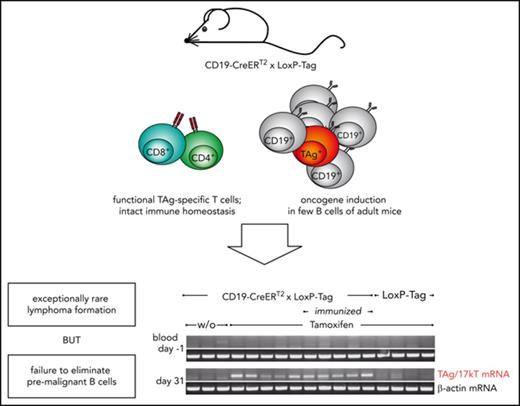
In order to investigate the T-cell response to an emerging B-cell neoplasia, we took advantage of the LoxP-Tag mouse model, which harbors an inducible SV40 large T-antigen (TAg).40,41 TAg is a well-characterized oncogene with defined class I epitopes42 and has been demonstrated to transform B cells.43,44 By crossing the LoxP-Tag mouse to CD19-Cre45 and CD19-CreERT2 35 mice, we generated 2 mouse models that differ in time and frequency of oncogene induction. Our results show that premalignant lymphoma-initiating B cells are not eradicated but do not progress in the autochthonous host. Lymphoma-initiating B cells rapidly progress, if T cells are lacking or T-cell homeostasis is otherwise perturbed.
Materials and methods
Mice
CD19-Cre45 and CD19-CreERT2 35 mice were bred to LoxP-Tag40 or Rosa-tdRFP46 mice. The F1 generation was analyzed. CD19-CreERT2 × LoxP-Tag mice were bred to OT-I × Thy1.1 [C57BL/6-Tg (TcraTcrb)1100Mjb × B6.PL-Thy1a/CyJ], Vil-Cre [B6.SJL-Tg (Vil-cre) 997 Gum/J, The Jackson Laboratory], and Rosa-tdRFP mice. Mice were heterozygous for all transgenes. Two- to 8-month-old CD4−/− (B6.129S2-Cd4tm1Mak, The Jackson Laboratory), CD8−/− (B6.129S2-Cd8atm1MaK, The Jackson Laboratory), Rag−/− (B6.129S6-Rag2tm1FwaN12, Taconic or B6.129S7Rag1tm1Mom/J; The Jackson Laboratory), and Rag2−/− × γc−/− (B10;B6-Rag2tm1FwaIl2rgtm1Wjl,Taconic) served as recipient mice, and C57Bl/6 or CD45.1 (B6.SJL-Ptprca Pepcb/BoyJ) mice as wild-type (wt) controls. T cells of P14 × Rag1−/− [B6.Cg-Tcratm1Mom Tg(TcrLCMV)327Sdz, backcrossed to Rag1−/−] mice were used for in vitro experiments.
All mice were on C57BL/6 background and bred and maintained under specific pathogen-free conditions in our animal facilities. Animal experiments were performed according to institutional and national guidelines and with approval of the Landesamt für Gesundheit und Soziales, Berlin, Germany.
Tamoxifen treatment
Tamoxifen (2 mg; Sigma) in olive oil/5% ethanol was administered orally by gavage on 5 consecutive days.
Lymphoma experiments
For survival analysis, mice were euthanized and subjected to full necropsy when a tumor was palpated or mice showed other signs of distress. Mice euthanized because of nontumor-related signs of distress were censored. For analysis of lymphoma onset, mice with >1% TAg+ cells of CD19+ cells (endogenous lymphoma formation) or >1% TAg+ CD19+ cells of lymphocytes (transfer experiments) in blood or spleen were classified as lymphoma bearing.
For lymphoma transplantation, splenocytes of lymphoma bearing CD19-Cre × LoxP-Tag mice or Rag−/− passaged lymphoma cells (supplemental Methods, available on the Blood Web site) were injected IV into recipient mice.
Transfer experiments
Splenocytes of young, untreated donor mice were pooled and injected directly or enriched for B cells by CD19+ magnetic-activated cell sorting (MACS; CD19 MicroBeads, Miltenyi Biotec). OT-I Thy1.1 CD8+ cells were enriched with the CD8a+ T Cell Isolation Kit (Miltenyi Biotec). In order to inject untouched CD4+/CD8+ T cells from CD19-Cre × LoxP-Tag and CD19-CreERT2 × LoxP-Tag donor mice, splenocytes were depleted of B cells by CD19+ MACS. B cells were injected (1 x 107 per recipient), and total cell numbers were adjusted accordingly. Residual contaminating T cells were depleted in vivo (supplemental Methods). Tamoxifen was applied from day 1 to day 5 after cell transfer whenever indicated.
For transfer of premalignant B cells, CD19-CreERT2 × LoxP-Tag mice were treated with tamoxifen (days 1-4). Splenocytes were CD19+ MACS sorted and 1 × 107 B cells of 1 donor were injected IV into 1 Rag−/− recipient. To inject splenocytes, the positive and negative MACS fraction of each donor mouse was combined according to the ratio of cells before MACS separation (containing 1 × 107 B cells, 3.5 × 106 CD8+ T cells, and 4.6-5.8 × 106 CD4+ T cells).
T-cell analyses
In vivo cytotoxicity assay.
Mice were immunized intraperitoneally (IP) with 1 × 107 TAg+ tumor cells (16.11340 ) whenever indicated. Naive wt control mice were left untreated. One week later, wt splenocytes were either nonloaded or loaded with 4 μg/mL H2-Kb restricted TAg-specific peptide IV (modified peptide VVYDFLKL, JPT Peptide Technologies GmbH), labeled with different amounts of carboxyfluorescein diacetate succinimidyl ester (CFSE; 0.1 μM and 1 μM) and mixed in equal parts. Target cells (2 × 107) were injected IV into each recipient. Eighteen to 24 h later, the presence of CFSE-labeled cells in spleens of recipient mice was analyzed by fluorescence-activated cell sorting (FACS). The ratio of peptide IV loaded cells to nonloaded cells was determined, and percentage of specific killing was calculated as follows: 100 − (ratio of challenged mice/ratio of naive control mice) × 100.
In vitro recognition assay.
T cells of P14 × Rag1−/− mice were transduced with the TAg epitope IV–specific T-cell receptor (TCR-IV) as described previously.47 Target cells were isolated from spleens of tamoxifen-treated or untreated mice, CD19+ MACS purified, and stimulated with 5 µM cytosine guanine dinucleotide ('5-TCCATGACGTTCCTGACGTT-3′, TIB Molbiol) overnight. B cells (5 × 105) and T cells (1 × 105) were cocultured for 3 days, and interferon γ (IFN-γ) levels in the supernatant were measured by enzyme-linked immunosorbent assay (ELISA; BD OptEIA, Mouse IFN-γ ELISA Set, BD Bioscience).
Tumor rejection analysis.
TAg+ tumor cells (1 × 106; 16.113) or Rag passaged lymphoma cells were injected subcutaneously (SC) or IV, respectively. The volume of subcutaneous tumors was calculated as follows: (π/6 × length × height × width). Outgrowth of lymphoma cells was analyzed in blood and by palpation of mice.
T-cell restimulation.
Splenocytes (5 × 106) were cultured in medium alone, 4 µg/mL TAg peptide IV (modified peptide VVYDFLKL; JPT Peptide Technologies GmbH), 10 ng/mL PMA (Calbiochem)/500 ng/mL Ionomycin (Calbiochem) or cocultured with 1 × 106 Rag passaged lymphoma cells for 5.5 to 10.5 h. After 1.5 h, 2.5 µg/mL Brefeldin A (Sigma) and 2.5 µg/mL Monensin (Sigma) were added to the culture. IFN-γ, CD4, and CD8 were stained intracellularly.
Histology
Organs were fixed in 10% formalin solution (4% formaldehyde, Sigma) and paraffin embedded, and 1-µm sections were stained with hematoxylin and eosin. For immunohistochemistry, sections were incubated with PAX5 (1H9), Ki67 (TEC-3), FOXP3 (FJK-16s), IRF4 (polyclonal, Cloud-Clone-Corp), BCL6 (IG191E/A8) antibodies, or peanut agglutinin (Vector Laboratories), after heat-induced epitope retrieval. For detection, Labeled Streptavidin-Biotin method or Dako REAL EnVision Detection System were used. Nuclear counterstaining was performed with hematoxylin.
Flow cytometry
The following antibodies were obtained from BD Pharmingen, eBioscience, or Biolegend: CD16/CD32 (2.4G2), B220 (RA3-6B2), CD19 (1D3 or 6D5), CD43 (S7), immunoglobulin M (IgM; II/41), IgD (11-26c.2a), CD23 (B3B4), CD21 (7G6), CD5 (53-7.3), CD8 (53-6.7), CD4 (RM4-5), CD44 (IM7.8.1 or IM7), CD86 (GL-1), Kb (AF6-88.5), CD178 (MFL3), I-Ab (AF6-120.1), CD40 (HM40-3), CD95 (Jo2), CD80 (16-10A1), IFN-γ (XMG1.2), and SV40 large T/small T (PAb 108). TAg peptide IV–specific CD8+ T cells were stained with iTAg Custom Tetramer/PE–H-2 Kb (modified peptide VVYDFLKL; MBL International Corporation). IgM and IFN-γ were stained with intracellular staining buffers (Biolegend). SV40 large T/small T was stained with the FoxP-3 staining Kit (eBioscience). Dead cells were excluded with 7AAD (Biolegend) or fixable viability dye eFluor 660 or eFluor450 (eBioscience). Analytical samples were acquired with the BD FACSCalibur (BD Bioscience), BD LSRFortessa (BD Bioscience), or MacsQuant Analyzer (Miltenyi Biotec), and data were analyzed with FlowJo software (FlowJo LLC). Cell sorting was performed on a FACSAria III (BD Bioscience).
DNA and RNA analyses
DNA and RNA of cells were isolated with the NucleoSpinTriPrepKit (Macherey-Nagel). Complementary DNA was synthesized with the Advantage reverse transcription–polymerase chain reaction (PCR) kit (Clontech). TAg expression was analyzed amplifying a 252-bp fragment of TAg and 17kT. Cre-mediated recombination and deletion of the stop cassette (ΔDNA) was detected by amplifying a 114-bp fragment. Amplification of β-actin served as control. Primer sequences are listed in the supplemental Methods.
Statistical analyses
All statistical analyses were performed using GraphPad Prism version 7.01 for Windows (GraphPad Software, La Jolla, CA).
Results
Generation of B-cell lymphoma models
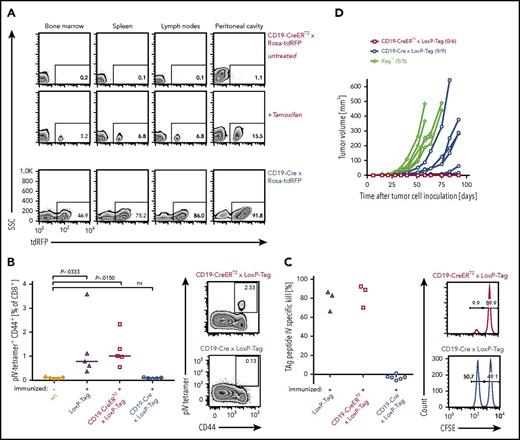
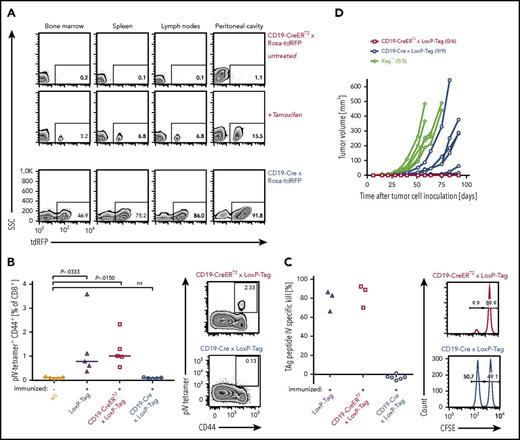
Young LoxP-Tag mice carry TAg as inducible oncogene and contain functional cytotoxic T lymphocytes (CTLs) specific for the dominant epitope IV of TAg.40,41 By crossing LoxP-Tag mice to either CD19-Cre or CD19-CreERT2 mice, TAg expression is specifically switched on in the B-cell lineage by Cre/loxP-mediated recombination. In CD19-Cre × LoxP-Tag mice the Cre-recombinase is constitutively active, whereas Cre-activity in CD19-CreERT2 × LoxP-Tag mice is tamoxifen dependent and can be induced in the adult host. With the help of red fluorescent protein (RFP) reporter mice,46 the recombination frequency of both Cre strains was analyzed, showing on average 74% and 7% RFP+ B cells in the spleen of CD19-Cre × Rosa-tdRFP and tamoxifen-treated CD19-CreERT2 × Rosa-tdRFP mice, respectively (Figure 1A). The frequency of RFP+ B cells was lower in the bone marrow (44% and 4%) and elevated in the peritoneal cavity (88% and 14%). No RFP+ cells were detected without tamoxifen application in CD19-CreERT2 × Rosa-tdRFP mice or in the CD19− cell fraction of the analyzed tissues. The recombination frequency in CD19-Cre × LoxP-Tag and CD19-CreERT2 × LoxP-Tag mice can only be inferred, because TAg and RFP transgenes are inserted into different genomic loci.48
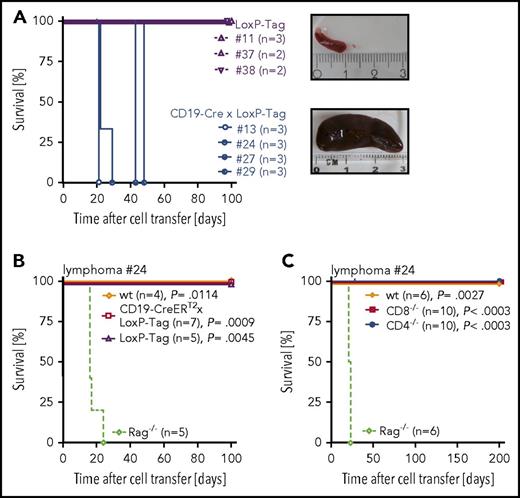
Characterization of oncogene-specific immunocompetence in nonviral B-cell lymphoma models. (A) Tissue-specific recombination pattern of CD19-CreERT2 and CD19-Cre mice. Three-month-old tamoxifen-treated (n = 3) or untreated (n = 3) CD19-CreERT2 × Rosa-tdRFP mice were analyzed for RFP expression in indicated tissues on day 15. CD19-Cre × Rosa-tdRFP mice (n = 5) were left untreated and analyzed at 6 weeks of age. Frequencies of RFP+ cells (gated on CD19+ lymphocytes) are indicated on representative flow cytometry plots. Lymph node samples include mesenteric, inguinal, and axillary lymph nodes. (B) TAg pIV–tetramer analysis. Ten- to 12-week-old LoxP-Tag (n = 5), CD19-CreERT2 × LoxP-Tag (n = 5), and CD19-Cre × LoxP-Tag (n = 5) mice were immunized IP with TAg+ tumor cells (16.113) and analyzed 1 week later for the presence of TAg-specific pIV-tetramer+ CD44+ CD8+ T cells in the spleen. Untreated wt mice (n = 5) served as negative control. Data summary (left panel) and representative FACS plots (right panel) are shown. Kruskal-Wallis test with Dunn’s post hoc test were performed. Adjusted P values are indicated. (C) In vivo cytotoxicity assay. Eight- to 10-week-old CD19-Cre × LoxP-Tag (n = 6), CD19-CreERT2 × LoxP-Tag (n = 3), and LoxP-Tag (n = 3) control littermates were immunized with TAg+ tumor cells (16.113) and challenged 1 week later with a mixture of TAg peptide IV loaded (CFSE low) and nonloaded target cells (CFSE high). The ratio of both populations was determined, and percentage of specific killing was calculated. Histograms gated on all CFSE+ cells are shown. Data are representative for 2 experiments. (D) Tumor rejection analysis. TAg+ cancer cells (1 × 106; 16.113) were injected SC into 10- to 11-week-old CD19-CreERT2 × LoxP-Tag (n = 6) and CD19-Cre × LoxP-Tag (n = 9) mice, and tumor growth was monitored. Immunodeficient Rag−/− mice (n = 5) served as positive controls. Indicated numbers represent mice with palpable tumor burden of total mice per group. Data are representative for 2 experiments.
Characterization of oncogene-specific immunocompetence in nonviral B-cell lymphoma models. (A) Tissue-specific recombination pattern of CD19-CreERT2 and CD19-Cre mice. Three-month-old tamoxifen-treated (n = 3) or untreated (n = 3) CD19-CreERT2 × Rosa-tdRFP mice were analyzed for RFP expression in indicated tissues on day 15. CD19-Cre × Rosa-tdRFP mice (n = 5) were left untreated and analyzed at 6 weeks of age. Frequencies of RFP+ cells (gated on CD19+ lymphocytes) are indicated on representative flow cytometry plots. Lymph node samples include mesenteric, inguinal, and axillary lymph nodes. (B) TAg pIV–tetramer analysis. Ten- to 12-week-old LoxP-Tag (n = 5), CD19-CreERT2 × LoxP-Tag (n = 5), and CD19-Cre × LoxP-Tag (n = 5) mice were immunized IP with TAg+ tumor cells (16.113) and analyzed 1 week later for the presence of TAg-specific pIV-tetramer+ CD44+ CD8+ T cells in the spleen. Untreated wt mice (n = 5) served as negative control. Data summary (left panel) and representative FACS plots (right panel) are shown. Kruskal-Wallis test with Dunn’s post hoc test were performed. Adjusted P values are indicated. (C) In vivo cytotoxicity assay. Eight- to 10-week-old CD19-Cre × LoxP-Tag (n = 6), CD19-CreERT2 × LoxP-Tag (n = 3), and LoxP-Tag (n = 3) control littermates were immunized with TAg+ tumor cells (16.113) and challenged 1 week later with a mixture of TAg peptide IV loaded (CFSE low) and nonloaded target cells (CFSE high). The ratio of both populations was determined, and percentage of specific killing was calculated. Histograms gated on all CFSE+ cells are shown. Data are representative for 2 experiments. (D) Tumor rejection analysis. TAg+ cancer cells (1 × 106; 16.113) were injected SC into 10- to 11-week-old CD19-CreERT2 × LoxP-Tag (n = 6) and CD19-Cre × LoxP-Tag (n = 9) mice, and tumor growth was monitored. Immunodeficient Rag−/− mice (n = 5) served as positive controls. Indicated numbers represent mice with palpable tumor burden of total mice per group. Data are representative for 2 experiments.
In order to assess the ability of CD19-Cre × LoxP-Tag and CD19-CreERT2 × LoxP-Tag mice to raise a TAg-specific CD8+ T-cell response, tetramer analysis and in vivo cytotoxicity assays were used. Young noninduced CD19-CreERT2 × LoxP-Tag mice retained functional TAg epitope IV–specific CTLs (Figure 1B) with cell lysing capacity (Figure 1C). In contrast, the CD8+ T-cell compartment of young CD19-Cre × LoxP-Tag mice was TAg tolerant (Figure 1B-C). Accordingly, injection of TAg-expressing 16.113 cancer cell suspensions SC led to cancer cell outgrowth in CD19-Cre × LoxP-Tag mice and cancer cell rejection in CD19-CreERT2 × LoxP-Tag mice (Figure 1D).
CD19-Cre × LoxP-Tag but not CD19-CreERT2 × LoxP-Tag mice develop B-cell lymphomas
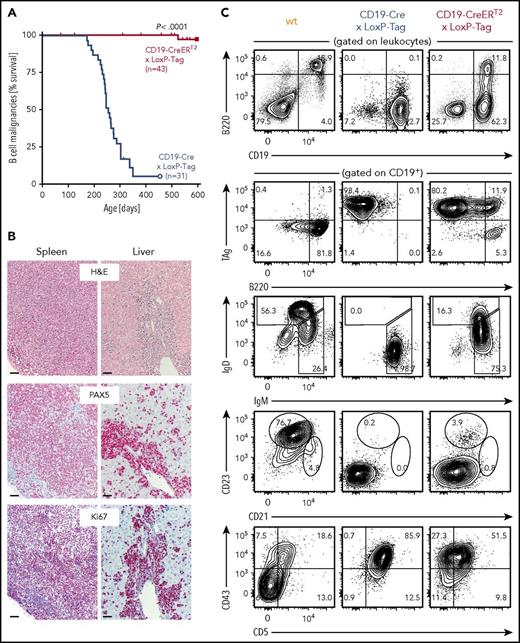
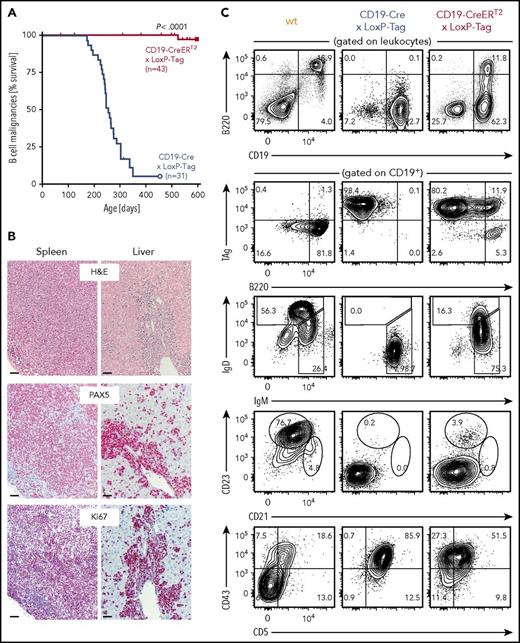
Despite the high recombination frequency in CD19-Cre × Rosa-tdRFP mice, appearance and expansion of TAg+ B cells in CD19-Cre × LoxP-Tag mice progressed slowly (supplemental Figure 1) but with high penetrance. At 8 months of age, the probability to present with B-cell lymphoma and symptoms of splenomegaly, often combined with hepatomegaly and enlarged thymus, lymph nodes, or pancreas was 50% for CD19-Cre × LoxP-Tag mice (Figure 2A). In contrast, tamoxifen-induced CD19-CreERT2 × LoxP-Tag mice developed lymphomas exceptionally rare and late in life (1 out of 43 mice at 17 months of age). Stochastic formation of non-B-cell tumors occurred in a similar time frame and was likely caused by Cre-independent deletions and read-through mechanisms of the LoxP-Tag transgene.40 The shift in average tumor latency between first-generation LoxP-Tag mice (6 month) and following generations of LoxP-Tag mice (14 month) was delayed even further for unknown reasons.
Rare development of B-cell lymphomas in CD19-CreERT2× LoxP-Tag mice. (A) Kaplan-Meier survival graph of B-cell malignancies in CD19-Cre × LoxP-Tag and tamoxifen-treated CD19-CreERT2 × LoxP-Tag mice. Tamoxifen was given at 6 to 8.5 weeks of age. Mice euthanized because of formation of non-B-cell tumor entities (n = 12) or other signs of distress (n = 4) were censored. CD19-CreERT2 × LoxP-Tag mice were monitored until day 951, but no further B-cell tumor was detected. The log-rank test was performed. (B) Spleen and liver sections of tumor bearing CD19-Cre × LoxP-Tag (n = 12) and CD19-CreERT2 × LoxP-Tag mice (n = 2) were stained for hematoxylin and eosin (H&E), PAX5, and Ki67. Shown are sections of 1 representative mouse. Bars represent 50 µm. (C) Splenocytes of tumor-bearing CD19-Cre × LoxP-Tag (n = 12) and CD19-CreERT2 × LoxP-Tag mice (n = 3) or wt control (n = 1) were analyzed by flow cytometry for B-cell linage markers and TAg expression. FACS plots of representative mice are depicted.
Rare development of B-cell lymphomas in CD19-CreERT2× LoxP-Tag mice. (A) Kaplan-Meier survival graph of B-cell malignancies in CD19-Cre × LoxP-Tag and tamoxifen-treated CD19-CreERT2 × LoxP-Tag mice. Tamoxifen was given at 6 to 8.5 weeks of age. Mice euthanized because of formation of non-B-cell tumor entities (n = 12) or other signs of distress (n = 4) were censored. CD19-CreERT2 × LoxP-Tag mice were monitored until day 951, but no further B-cell tumor was detected. The log-rank test was performed. (B) Spleen and liver sections of tumor bearing CD19-Cre × LoxP-Tag (n = 12) and CD19-CreERT2 × LoxP-Tag mice (n = 2) were stained for hematoxylin and eosin (H&E), PAX5, and Ki67. Shown are sections of 1 representative mouse. Bars represent 50 µm. (C) Splenocytes of tumor-bearing CD19-Cre × LoxP-Tag (n = 12) and CD19-CreERT2 × LoxP-Tag mice (n = 3) or wt control (n = 1) were analyzed by flow cytometry for B-cell linage markers and TAg expression. FACS plots of representative mice are depicted.
Pathological evaluation of spleen and liver sections of tumor bearing CD19-Cre × LoxP-Tag and CD19-CreERT2 × LoxP-Tag mice showed medium- to large-sized lymphoid cells with diffuse growth pattern (Figure 2B). The tumors stained positive for the transcription factor PAX5 that serves as diagnostic marker for B-cell lineage determination in human lymphoid malignancies. The tumor cells displayed a high Ki67 index of >50% and did not express germinal center markers (supplemental Figure 2). Sequencing analysis detected no somatic mutations in the rearranged IgH genes, but instead VH11-DH1/DH2-JH1 rearrangements and CDR3 sequences typical for B1a cells (supplemental Table 1).49 The lymphoma was classified as high-grade B-cell lymphoma accordingly. Further analysis of cell lineage markers characterized the lymphoma primarily as CD19+ B220low CD43+ CD5+ IgM+ (Figure 2C; supplemental Table 2), again suggesting B1a cells as predominant lymphoma origin. They expressed CD44, CD80, CD40, CD86, and MHC class I and class II, whereas Fas and Fas ligand expression appeared to be low or absent (supplemental Figure 3). The lymphomas were clonal or oligoclonal of origin (supplemental Figure 4).
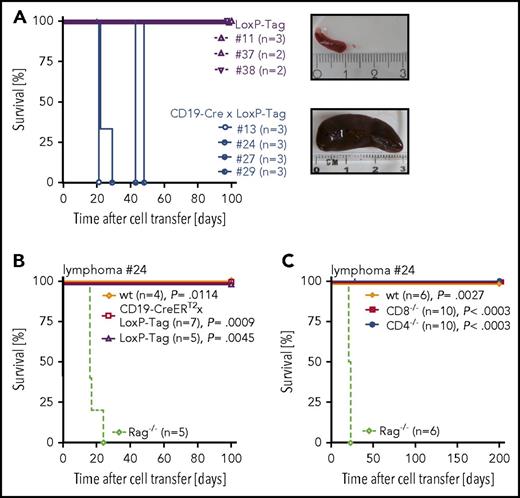
Transfer of TAg+ B lymphoma cells into immunocompromised Rag2−/− × γc−/− mice demonstrated their tumorigenicity (Figure 3A). Rejection of lymphoma cells in young LoxP-Tag, CD19-CreERT2 × LoxP-Tag, wt, CD4−/−, and CD8−/− mice showed the immunogenicity of transplanted TAg+ lymphoma cells (Figure 3B-C; supplemental Figure 5) and indicated a role for both CD8+ and CD4+ T cells in lymphoma-cell rejection.
Transplanted TAg+B cells are tumorigenic and immunogenic. (A) Splenocytes (1 × 106) of lymphoma bearing CD19-Cre × LoxP-Tag mice (n = 4) or age-matched tumor free LoxP-Tag control littermates (n = 3) were injected IV into Rag2−/− × γc−/− mice. Recipient mice were euthanized when lymphoma burden was palpable. Tumor burden manifested mainly as splenomegaly, depicted as exemplary images of a lymphoma-free (upper panel) and lymphoma-bearing (lower panel) Rag2−/− × γc−/− recipient. (B-C) Splenocytes (1 × 106) of Rag passaged lymphoma cells were injected IV into indicated recipient mice. Recipient mice were euthanized when tumor burden was palpable. Shown are combined data of 2 independent experiments for 1 out of 2 representative lymphomas (#24) isolated from CD19-Cre × LoxP-Tag mice. The log-rank test and Bonferroni post hoc test were performed. Adjusted P values are indicated.
Transplanted TAg+B cells are tumorigenic and immunogenic. (A) Splenocytes (1 × 106) of lymphoma bearing CD19-Cre × LoxP-Tag mice (n = 4) or age-matched tumor free LoxP-Tag control littermates (n = 3) were injected IV into Rag2−/− × γc−/− mice. Recipient mice were euthanized when lymphoma burden was palpable. Tumor burden manifested mainly as splenomegaly, depicted as exemplary images of a lymphoma-free (upper panel) and lymphoma-bearing (lower panel) Rag2−/− × γc−/− recipient. (B-C) Splenocytes (1 × 106) of Rag passaged lymphoma cells were injected IV into indicated recipient mice. Recipient mice were euthanized when tumor burden was palpable. Shown are combined data of 2 independent experiments for 1 out of 2 representative lymphomas (#24) isolated from CD19-Cre × LoxP-Tag mice. The log-rank test and Bonferroni post hoc test were performed. Adjusted P values are indicated.
Increased lymphoma incidence in the absence of TAg-specific T cells
The exceptionally low incidence of lymphoma formation observed in CD19-CreERT2 × LoxP-Tag mice raised the question of whether the low recombination frequency, oncogene induction in the adult host, or presence of TAg-competent T cells accounts for this phenomenon.
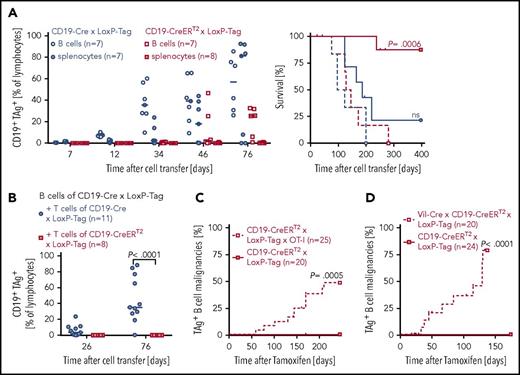
To investigate the impact of CD8+ and CD4+ T cells on lymphoma formation, comparative analyses of lymphoma incidence in the presence and absence of T cells were conducted. B cells or splenocytes of CD19-CreERT2 × LoxP-Tag mice were transferred into immunodeficient Rag−/− mice, and oncogene expression was induced in the recipient mouse. In the absence of T cells, B cells of CD19-CreERT2 × LoxP-Tag mice formed TAg+ B-cell lymphomas in all recipient mice in a comparable time frame to CD19-Cre × LoxP-Tag B cells, despite their differences in recombination frequency (Figure 4A). Cotransfer of oncogene-unspecific OT-I CD8+ T cells did not prevent lymphoma outgrowth (supplemental Figure 6). However, in the presence of TAg-competent T cells, only 1 out of 8 recipient mice developed a TAg+ B-cell lymphoma. Oncogene-specificity of protective T cells was addressed by cotransfer of TAg-competent or TAg-tolerant T cells. B cells of CD19-Cre × LoxP-Tag mice were transferred IV into Rag−/− recipients. Splenocytes of TAg-competent CD19-CreERT2 × LoxP-Tag or TAg-tolerant CD19-Cre × LoxP-Tag mice were depleted of B cells and injected into the contralateral tail vein. Only mice that received TAg-competent splenocytes remained tumor free (Figure 4B).
Outgrowth of TAg+B-cell lymphomas in the absence of TAg-competent T cells. (A) B cells (open symbols) or splenocytes (closed symbols) of CD19-CreERT2 × LoxP-Tag and CD19-Cre × LoxP-Tag mice were transferred IV into Rag−/− mice. Each recipient obtained 1 × 107 B cells. To deplete residual T cells, α-Thy1.2 antibodies or isotype control was injected twice (day 0 and day 5). Tamoxifen was applied on days 1 to 5 after transfer. Blood samples were stained for TAg expression (left), and mice with palpable lymphoma burden were euthanized and presented as Kaplan-Meier survival graph (right). Mice showing TAg-unrelated signs of distress were censored. The log-rank test and Bonferroni post hoc test were performed. Adjusted P values are indicated. (B) B cells (1 × 107) of CD19-Cre × LoxP-Tag mice were transferred IV into Rag−/− recipients. Splenocytes of TAg-competent CD19-CreERT2 × LoxP-Tag or TAg-tolerant CD19-Cre × LoxP-Tag mice were depleted for B cells and injected into the contralateral tail vein (5 × 106 CD8+ T cells and 6-10 × 106 CD4+ T cells). Blood samples were stained for TAg+ B cells to monitor lymphoma formation. Two-tailed Mann-Whitney U test was applied. Kaplan-Meier survival graph of tamoxifen-treated CD19-CreERT2 × LoxP-Tag × OT-I and CD19-CreERT2 × LoxP-Tag mice (10/20 cohoused control littermates, 10/20 separate breeding) (C) and Vil-Cre × CD19-CreERT2 × LoxP-Tag mice and cohoused CD19-CreERT2 × LoxP-Tag control littermates (D). Tamoxifen was given at 6 to 10 weeks of age. Censored mice were euthanized because of formation of other tumor entities or other signs of distress. The log-rank test was performed.
Outgrowth of TAg+B-cell lymphomas in the absence of TAg-competent T cells. (A) B cells (open symbols) or splenocytes (closed symbols) of CD19-CreERT2 × LoxP-Tag and CD19-Cre × LoxP-Tag mice were transferred IV into Rag−/− mice. Each recipient obtained 1 × 107 B cells. To deplete residual T cells, α-Thy1.2 antibodies or isotype control was injected twice (day 0 and day 5). Tamoxifen was applied on days 1 to 5 after transfer. Blood samples were stained for TAg expression (left), and mice with palpable lymphoma burden were euthanized and presented as Kaplan-Meier survival graph (right). Mice showing TAg-unrelated signs of distress were censored. The log-rank test and Bonferroni post hoc test were performed. Adjusted P values are indicated. (B) B cells (1 × 107) of CD19-Cre × LoxP-Tag mice were transferred IV into Rag−/− recipients. Splenocytes of TAg-competent CD19-CreERT2 × LoxP-Tag or TAg-tolerant CD19-Cre × LoxP-Tag mice were depleted for B cells and injected into the contralateral tail vein (5 × 106 CD8+ T cells and 6-10 × 106 CD4+ T cells). Blood samples were stained for TAg+ B cells to monitor lymphoma formation. Two-tailed Mann-Whitney U test was applied. Kaplan-Meier survival graph of tamoxifen-treated CD19-CreERT2 × LoxP-Tag × OT-I and CD19-CreERT2 × LoxP-Tag mice (10/20 cohoused control littermates, 10/20 separate breeding) (C) and Vil-Cre × CD19-CreERT2 × LoxP-Tag mice and cohoused CD19-CreERT2 × LoxP-Tag control littermates (D). Tamoxifen was given at 6 to 10 weeks of age. Censored mice were euthanized because of formation of other tumor entities or other signs of distress. The log-rank test was performed.
To verify these data in the autochthonous host, we bred CD19-CreERT2 × LoxP-Tag mice to OT-I mice. CD19-CreERT2 × LoxP-Tag × OT-I mice allow the induction of B-cell-specific TAg expression in an environment of monoclonal oncogene-unspecific OT-I CD8+ T cells. The expression of the class I restricted ovalbumin-specific TCR strongly skews the T-cell compartment to the CD8+ T-cell subtype, and mice harbor only few CD4+ T cells.50 The probability for CD19-CreERT2 × LoxP-Tag × OT-I mice to develop >1% TAg+ B cells in blood or spleen within 8 months after tamoxifen application was 48% (Figure 4C). In none of the CD19-CreERT2 × LoxP-Tag control mice were TAg+ B cells detected in this time frame. An increased probability to develop B-cell lymphomas was also observed in the presence of polyclonal but TAg-tolerant T cells. Vil-Cre × CD19-CreERT2 × LoxP-Tag mice retain the quality of inducible TAg expression in B cells, but early thymic and gastrointestinal expression of Vil-Cre leads to a TAg-tolerant CD8+ T-cell compartment.51,52 Coincident with rapid onset of tumors in the gastrointestinal tract, pancreas, kidney, and thymus (19/20), TAg+ B cells were detected in blood or spleen in 9 out of 20 mice within 175 days after tamoxifen application (Figure 4D). Two TAg+ lymphomas developed in CD19-CreERT2 × LoxP-Tag control littermates later in life (8 and 15 months after oncogene induction). Vil-Cre × LoxP-Tag mice did not show TAg+ B cells (this work and Czeh et al51 ).
Analysis of the endogenous TAg-specific CD8+ T-cell response
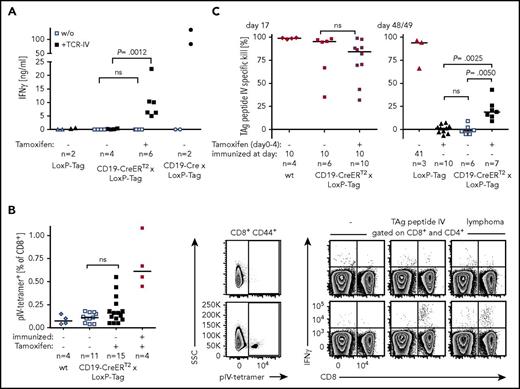
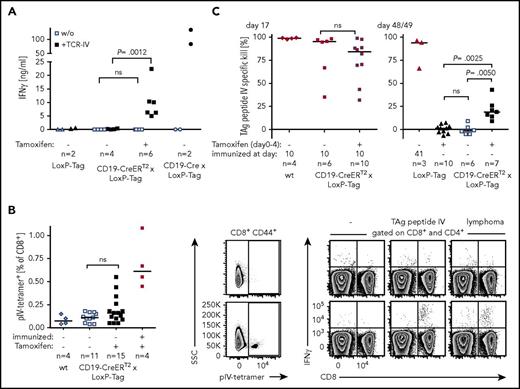
Data obtained in transplant experiments and transgenic mice with a skewed T-cell compartment suggested TAg-specific T-cell-mediated control of lymphoma outgrowth. However, the design of these experiments is inevitably linked to changes in the cell composition, the phenotype of cells, and/or the host milieu. The impact of these alterations is unknown. Consequently, direct analyses of the T-cell response in CD19-CreERT2 × LoxP-Tag mice were undertaken. TAg epitope IV has been described to be immunodominant and was the most promising target of a potential CD8+ T-cell response.42,53 To test whether or not tamoxifen-induced B cells are able to present TAg peptide IV to T cells in general, CD19+-enriched splenocytes of tamoxifen-treated or untreated mice were cocultured with TCR-IV. Secretion of IFN-γ demonstrated recognition by TAg-specific T cells in vitro (Figure 5A). Whether tamoxifen-induced B cells elicit a CD8+ T-cell response in vivo was analyzed by staining for TAg-peptide IV–specific CD8+ T cells in the spleens of mice and by in vivo cytotoxicity analysis. Young CD19-CreERT2 × LoxP-Tag mice were tamoxifen treated and analyzed for pIV-tetramer+ T cells 2 weeks later. Whereas no pIV-tetramer+ T cells could be detected in the majority of mice, few mice presented with pIV-tetramer+ T cells (Figure 5B, left and middle panel). Specificity of this response and T-cell function was confirmed by IFN-γ secretion in response to TAg peptide IV and TAg+ lymphoma cells (Figure 5B, right panel). No IFN-γ secretion was observed in the CD4+ T-cell compartment. In vivo cytotoxicity assays excluded the tolerization of TAg-specific CD8+ T cells after B cell-specific TAg induction (Figure 5C, left panel) but instead showed a weak TAg pIV–specific CTL response 1.5 months after tamoxifen application (Figure 5C, right panel).
Recognition of TAg+lymphoma cells by CD8+T cells. (A) B cells of 5- to 7-week-old tamoxifen-treated (day 0-4) or untreated mice were isolated on days 17 to 24, activated with cytosine guanine dinucleotide and cocultured with TCR-IV–transduced CD8+ T cells for 3 days. The IFN-γ concentration in the supernatant was determined by ELISA. Each dot represents the IFN-γ response to 1 mouse. Shown are combined data of 2 independent experiments. One-way analysis of variance and Sidak’s post hoc test were performed. Adjusted P values are indicated. (B) Nine- to 12-week-old CD19-CreERT2 × LoxP-Tag mice were tamoxifen treated (day 0-4) or left untouched. The positive control was immunized with 1 × 107 TAg+ tumor cells at day −7 (symbols in red). Untreated wt mice served as negative control. On day 14/15, splenocytes were stained with pIV-tetramer and were restimulated with medium (-), TAg peptide IV, or TAg+ lymphoma cells. Shown are exemplary FACS plots of tamoxifen-treated CD19-CreERT2 × LoxP-Tag mice, which stained pIV-tetramer− (upper row) and pIV-tetramer+ (lower row). Combined data of 3 independent experiments were analyzed with 1-tailed Mann-Whitney U test. (C) Six- to 9-week-old mice were tamoxifen treated or remained untouched. Mice were immunized IP with 1 × 107 TAg+ tumor cells on day 10 or 41 (symbols in red) or stayed nonimmunized. The TAg peptide IV–specific CTL response was evaluated by in vivo cytotoxicity assays on day 17 (left) or day 48/49 (right). Shown are combined data of 2 independent experiments. Two-tailed Mann-Whitney U test (left) and Kruskal-Wallis test with Dunn’s post hoc test (right) were performed. Adjusted P values are indicated.
Recognition of TAg+lymphoma cells by CD8+T cells. (A) B cells of 5- to 7-week-old tamoxifen-treated (day 0-4) or untreated mice were isolated on days 17 to 24, activated with cytosine guanine dinucleotide and cocultured with TCR-IV–transduced CD8+ T cells for 3 days. The IFN-γ concentration in the supernatant was determined by ELISA. Each dot represents the IFN-γ response to 1 mouse. Shown are combined data of 2 independent experiments. One-way analysis of variance and Sidak’s post hoc test were performed. Adjusted P values are indicated. (B) Nine- to 12-week-old CD19-CreERT2 × LoxP-Tag mice were tamoxifen treated (day 0-4) or left untouched. The positive control was immunized with 1 × 107 TAg+ tumor cells at day −7 (symbols in red). Untreated wt mice served as negative control. On day 14/15, splenocytes were stained with pIV-tetramer and were restimulated with medium (-), TAg peptide IV, or TAg+ lymphoma cells. Shown are exemplary FACS plots of tamoxifen-treated CD19-CreERT2 × LoxP-Tag mice, which stained pIV-tetramer− (upper row) and pIV-tetramer+ (lower row). Combined data of 3 independent experiments were analyzed with 1-tailed Mann-Whitney U test. (C) Six- to 9-week-old mice were tamoxifen treated or remained untouched. Mice were immunized IP with 1 × 107 TAg+ tumor cells on day 10 or 41 (symbols in red) or stayed nonimmunized. The TAg peptide IV–specific CTL response was evaluated by in vivo cytotoxicity assays on day 17 (left) or day 48/49 (right). Shown are combined data of 2 independent experiments. Two-tailed Mann-Whitney U test (left) and Kruskal-Wallis test with Dunn’s post hoc test (right) were performed. Adjusted P values are indicated.
T cells fail to eradicate lymphoma-initiating cells
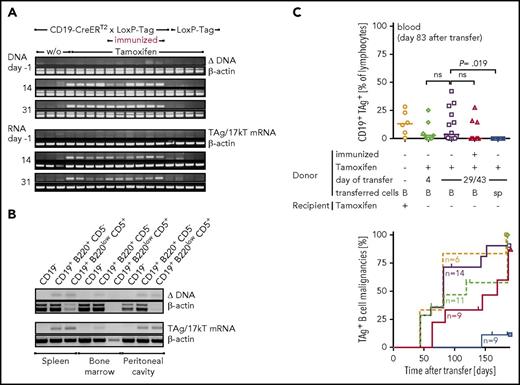
Despite the exceptional rare lymphoma development, both deletion of the stop cassette and TAg messenger RNA, could be detected in blood cells of all tamoxifen-treated CD19-CreERT2 × LoxP-Tag mice over time (Figure 6A). Noninduced mice or LoxP-Tag control littermates remained negative. Mice that had been immunized with TAg-positive cancer cells 1 week prior to oncogene induction also retained TAg-expressing cells, indicating the failure of removal of TAg-initiated B cells despite an induced immune response by immunization. Further analyses revealed persistence of TAg-initiated B cells also in the bone marrow, spleen, and peritoneal cavity (Figure 6B). Phenotypic characterization of these premalignant B cells was performed in CD19-CreERT2 × LoxP-Tag × Rosa-tdRFP mice using RFP expression as surrogate marker for recombination (supplemental Figure 7). Here, the proportion of cells showing recombination was slightly elevated in the CD19+ B220low CD5+ compartment of the peritoneal cavity, and RFP+ cells demonstrated expression of MHC class I, MHC class II, and CD40.
TAg-specific T cells fail to eradicate lymphoma-initiating cells. (A) Nine- to 11-week-old CD19-CreERT2 × LoxP-Tag mice were tamoxifen treated (n = 5) or left untreated (n = 3). One group was immunized IP with 1 × 107 TAg+ cancer cells on day −7 and subsequently tamoxifen treated (n = 5). LoxP-Tag control littermates were also tamoxifen treated (n = 4). Tamoxifen was applied on days 0-4. PCRs specific for the Cre-mediated deletion and TAg RNA expression were performed from total blood cells. PCR for β-actin served as control. Data are representative for 2 independent experiments. (B) Twelve-week-old CD19-CreERT2 × LoxP-Tag mice were tamoxifen treated (day 0-4), and indicated organs were FACS sorted for CD19−, CD19+B220+CD5−, and CD19+B220lowCD5+ cells on days 30 to 49. Cell numbers differed between 6000 and 100 000 cells. PCRs specific for the Cre-mediated deletion and TAg RNA expression were performed. Representative data for n = 3. (C) B cells (B) or splenocytes (sp) of CD19-CreERT2 × LoxP-Tag mice were isolated shortly after (day 4) or long after (days 29-43) tamoxifen treatment (days 0-4, 7- to 10-week-old mice) and injected into Rag−/− mice. Each mouse received 1 × 107 B cells. One group was immunized with 1 × 107 TAg+ tumor cells 1 week prior to tamoxifen treatment (day −7, symbols in red). One group received tamoxifen after transfer in the recipient host. Shown are combined data of 2 independent experiments. Outgrowth of TAg+ B cells was analyzed in blood 83 days after transfer (upper graph). Kruskal-Wallis test and Dunn’s post hoc test were performed; adjusted P values are indicated. Mice with >1% TAg+ CD19+ cells in blood or spleen were considered lymphoma bearing, and lymphoma onset is depicted as Kaplan-Meier graph. Censored mice were euthanized because of other signs of distress.
TAg-specific T cells fail to eradicate lymphoma-initiating cells. (A) Nine- to 11-week-old CD19-CreERT2 × LoxP-Tag mice were tamoxifen treated (n = 5) or left untreated (n = 3). One group was immunized IP with 1 × 107 TAg+ cancer cells on day −7 and subsequently tamoxifen treated (n = 5). LoxP-Tag control littermates were also tamoxifen treated (n = 4). Tamoxifen was applied on days 0-4. PCRs specific for the Cre-mediated deletion and TAg RNA expression were performed from total blood cells. PCR for β-actin served as control. Data are representative for 2 independent experiments. (B) Twelve-week-old CD19-CreERT2 × LoxP-Tag mice were tamoxifen treated (day 0-4), and indicated organs were FACS sorted for CD19−, CD19+B220+CD5−, and CD19+B220lowCD5+ cells on days 30 to 49. Cell numbers differed between 6000 and 100 000 cells. PCRs specific for the Cre-mediated deletion and TAg RNA expression were performed. Representative data for n = 3. (C) B cells (B) or splenocytes (sp) of CD19-CreERT2 × LoxP-Tag mice were isolated shortly after (day 4) or long after (days 29-43) tamoxifen treatment (days 0-4, 7- to 10-week-old mice) and injected into Rag−/− mice. Each mouse received 1 × 107 B cells. One group was immunized with 1 × 107 TAg+ tumor cells 1 week prior to tamoxifen treatment (day −7, symbols in red). One group received tamoxifen after transfer in the recipient host. Shown are combined data of 2 independent experiments. Outgrowth of TAg+ B cells was analyzed in blood 83 days after transfer (upper graph). Kruskal-Wallis test and Dunn’s post hoc test were performed; adjusted P values are indicated. Mice with >1% TAg+ CD19+ cells in blood or spleen were considered lymphoma bearing, and lymphoma onset is depicted as Kaplan-Meier graph. Censored mice were euthanized because of other signs of distress.
In order to verify the lymphoma-forming potential of the premalignant B cells detected by PCR, we transferred B cells at different times after oncogene induction into immunocompromised Rag−/− mice. Outgrowth of TAg+ B-cell lymphomas confirmed persistence of premalignant lymphoma-initiating B cells in the primary host, even despite a TAg-specific T-cell response induced by immunization (Figure 6C). Analysis of IgH VDJ rearrangements of lymphomas proved their mono- to oligoclonal origin (supplemental Figure 8).
Discussion
To study the role of T cells during B-cell lymphoma development, we generated 2 different mouse models, both based on TAg as inducible oncogene and potential rejection antigen. CD19-Cre × LoxP-Tag mice were neonatal tolerant for TAg, deleted the stop cassette in many B cells (based on reporter gene activation), and developed lymphomas with high penetrance. In contrast, CD19-CreERT2 × LoxP-Tag mice had retained TAg-specific pIV T cells at young age, deleted the stop cassette in comparably few B cells following tamoxifen application, which better resembled sporadic lymphoma development, and rarely developed lymphomas. Thus, the question was whether and how T cells control B-cell lymphomas in the latter mouse line. The question is relevant because B cells efficiently present antigen through MHC I and MHC II molecules. Nevertheless, the role of B cells for T-cell priming has remained controversial.
It is critical to distinguish between virus-associated and sporadic B-cell lymphomas. In humans, T cells efficiently control Epstein-Barr virus–associated lymphomas as an essential evolutionary requirement.3 In mouse models transgenic for Epstein-Barr virus antigens LMP1 and LMP2, lymphoproliferative disease is also controlled by T cells.34,35,38 These antigens are unique because they have profound effects on the B-cell phenotype and mimic activation signals, which likely explains elimination of “activated” B cells by T cells, not necessarily LMP specific. We do not have any indication that TAg affects B-cell activation. Therefore, TAg expression appears to resemble a cellular, yet antigenic, antigen in CD19-CreERT2 × LoxP-Tag mice. However, TAg harbors several CD8+ and CD4+ T-cell epitopes. Sporadic human B-cell malignancies carry a low to intermediate mutational burden,54,55 and future studies addressing the T-cell response to point mutations, for example in MyD88,56 would therefore be very interesting.
The interpretation of our data is difficult. The straightforward interpretation suggests immunosurveillance of B-cell lymphomas according to Burnet’s hypothesis that somatic mutations can create neoantigens, mimicked here by stop-cassette deletion and TAg activation in the adult host, leading to elimination by T cells.57 Initiated B cells from CD19-CreERT2 × LoxP-Tag mice rapidly progressed upon transfer into an immune-deficient host, which in turn was prevented by cotransfer of T cells from the same mice. Thus, it was not the lower precursor frequency of initiated B cells that caused the failure of lymphoma development in the autochthonous host. In agreement, lymphomas developed in the autochthonous host that did not possess TAg-specific T cells (only OT-I CD8+ T cells or TAg-tolerant T cells). Because B cells are able to present antigen to CD4+ and CD8+ T cells, both T-cell populations might have an impact that needs to be separately analyzed in the future.
However, our data could also be explained in that initiated B cells of CD19-CreERT2 × LoxP-Tag mice only progressed when the immune homeostasis was perturbed. By manipulating the T-cell compartment in experimental cancer models, the immune homeostasis might be changed alongside, potentially providing a growth advantage to lymphoma cells.58 Transfer experiments in immune-deficient mice could lead to homeostatic T-cell expansion and changes in the composition of the lymphoid compartment. Skewing of the T-cell compartment in OT-I transgenic mice could cause changes in B-cell homeostasis or the microbiome, although studies addressing the impact on the microbiota are still lacking. Increased lymphoma development in Vil-Cre × CD19-CreERT2 × LoxP-Tag mice could also be explained by other tumors, which occur additionally in these mice, because it was shown that tumors at one site permit the growth of other tumors at a distant site.59,60
Thus, we cannot formally distinguish between immunosurveillance and alterations in the general host-homeostasis, microenvironment, or baseline inflammatory responses in our model. B cells that had deleted the stop cassette and expressed TAg were recognized by TAg-specific CD8+ T cells in vitro, yet in vivo they were not eliminated. Furthermore, in the majority of mice, no or only a weak TAg-specific CD8+ T-cell response was detected following tamoxifen-mediated TAg activation, but no B-cell lymphomas developed. It should be noted, however, that the usually clonal B-cell lymphomas had upregulated TAg expression. Elimination of such rare B-cell lymphoma progenitors by TAg-specific CD8+ T cells would have gone unnoticed by us. On the other hand, provided recombined TAg+ B cells persist and retain lymphoma-forming capacity lifelong, this possibility would be considered unlikely, because single LoxP-Tag mice develop Cre recombinase-independent sporadic tumors and TAg-specific tolerance later in life. In conclusion, our study suggests inhibition of premalignant B-cell lymphomas by T cells but at the same time demonstrates the failure of the immune system to eradicate these lymphoma-initiating B cells, retaining the risk for lymphoma development later in life.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank S. Nedospasov, M. Schmidt-Supprian, and H.-J. Fehling for providing CD19-Cre, CD19-CreERT2, and Rosa26-tdRFP mice, respectively. The authors also thank A. Textor for transduction of T cells and S. Horn, K. Borgwald, K. Retzlaff, J. Waldeck, and S. Spieckermann for excellent technical assistance.
This work was supported by grants of the Deutsche Forschungsgemeinschaft (TR54, SFB633 and TR36).
Authorship
Contribution: D.H. performed the experiments and analyzed the results; C.S. assisted in experimental design and execution; P.L. and A.A.K. performed histological stainings; P.L. and C.L. conducted tumor classification; T.S. supervised Southern blot analysis and performed PCR analysis of clonality; D.H., T.B., and G.W. designed the experiments and wrote the manuscript; and T.B. and G.W. conceived and supervised the project.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for P.L. is University Hospital Cologne, Institute of Pathology, Cologne, Germany.
Correspondence: Gerald Willimsky, Cooperation Unit for Experimental and Translational Cancer Immunology, Institute of Immunology (Charité - Universitätsmedizin Berlin) and German Cancer Research Center (DKFZ Heidelberg), Campus Berlin Buch, Lindenberger Weg 80, 13125 Berlin, Germany; e-mail: gerald.willimsky@charite.de.