Abstract
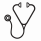
Although HCT offers the best potential for cure for patients with high risk leukemia and MDS, the procedure may not be an option for all patients due to the toxicity of the conditioning regimen. Reduced-intensity conditioning (RIC) allo-HCT regimens are associated with lower non-relapse mortality (NRM), but patients receiving these regimens have a higher risk of relapse. Radioimmunotherapy (RIT) can potentially deliver high doses of targeted radiation while minimizing toxicity to normal tissue. When combined with RIC allo-HCT, RIT may improve responses without increasing toxicity associated with myeloablative conditioning.
We evaluated the safety and efficacy of yttrium 90 (90Y)-anti-CD45 antibody (MAb; BC8) followed by a standard RIC regimen with fludarabine (Flu) and 2 Gy total body irradiation (TBI) as a means of developing an improved HCT strategy for high-risk acute leukemia or MDS patients. We used CD45 as a target due to its ubiquitous expression on hematopoietic stem cells including the leukemic blasts. We used the high-energy (2.2 MeVmax) beta-emitter 90Y, which has a relatively short half-life (2.7 days) to eliminate targeted malignant cells.
This phase I dose-escalation trial (NCT01300572) was designed to estimate the safety, feasibility and maximum tolerated dose (MTD) of 90Y-BC8-DOTA MAb when combined with Flu and 2 Gy TBI followed by HLA‐matched, related or unrelated allo-HCT for patients with high-risk leukemia or MDS. The MTD was defined as the radiation absorbed dose delivered by 90Y-BC8-DOTA MAb associated with a true dose-limiting toxicity (DLT) rate of 25%, where a DLT is defined as Bearman grade III/IV regimen-related toxicity. Doses of 90Y were escalated in increments of 2 Gy depending on the occurrence of DLT. Inclusion required patients to have advanced leukemia or high-risk MDS (defined as primary refractory or relapsed AML/ALL, secondary AML, MDS expressed as RAEB or CMML). To determine the dose of 90Y-BC8-DOTA, patients first underwent a biodistribution step using a trace-labeled infusion of 111Indium (111In)-BC8-DOTA followed by gamma-camera imaging. On pre-HCT day -12, patients received 90Y-BC8-DOTA at a prescribed radiation dose calculated from the trace-labeled 111In-BC8-DOTA biodistribution, followed by Flu (30 mg/m2/day) on days -4 to -2. TBI (2 Gy) was administered on day 0, prior to G-CSF mobilized donor PBSC infusion. GVHD prophylaxis consisted of mycophenolate mofetil and cyclosporine.
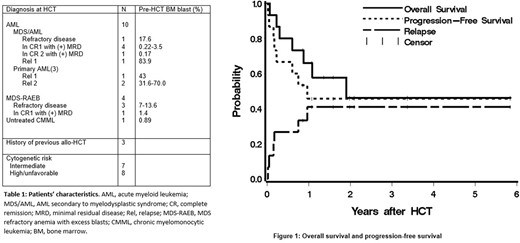
Fifteen patients, median age of 62 (range 37-76), were treated (10 with advanced AML, 5 with high-risk MDS). At time of HCT, 9 patients had refractory active disease while 6 were in remission with minimal residual disease (Table 1). The patients received 22.8 to 151.2 mCi of 90Y, delivering an average of 10.5 Gy to marrow, 70 Gy to spleen, and 17 Gy to liver. Although a maximum dose of 28 Gy was delivered to the liver, no DLT was observed. Therefore, the MTD could not be estimated.
Treatment led to complete remission in 13 patients (87%), 2 patients had persistent disease after HCT. All patients engrafted with a median donor-derived CD3 and CD33 chimerism both 100% by day 28 after HCT. Ten patients (67%) developed grade II-IV acute GVHD (grade II: n=7; III: n=2; IV: n=1). Five patients (33%) developed chronic GVHD. Six patients relapsed, 5 of whom subsequently died due to progression of disease. The median time to relapse among these 6 patients was 59 days (range, 6- 351 days). One patient died from stage IV steroid-refractory GVHD. One patient died in remission from acute renal failure at 7 months after HCT. Eight patients (53%) are surviving with a median follow-up of 1.8 (range, 0.9-5.9) years. Estimated overall survival at one and two years were 66% and 46%, respectively, with progression-free survival estimated to be 46% at each of these time points. The 1-year estimate of relapse was 41% (Fig. 1).
The inclusion of 90Y-BC8-DOTA into a RIC allo-HCT regimen is feasible, tolerable and no DLTs were observed. The efficacy of this approach is promising considering the high-risk leukemia/MDS patients with active disease enrolled. Current studies are evaluating the use of an alpha emitter, astatine-211, which is short-lived (t ½ = 7.2 hours) and provides high-energy radiation, conjugated to anti-CD45 MAb BC8 as part of an HCT conditioning regimen for patients with advanced AML, ALL, or high-risk MDS, in place of 90Y with the goal of continuing to improve outcomes using RIT for allo-HCT (NCT03128034).
Orozco:Actinium Pharmaceuticals: Research Funding. Green:Juno Therapeutics: Patents & Royalties, Research Funding. Gopal:Incyte: Consultancy; Pfizer: Research Funding; Gilead: Consultancy, Research Funding; Teva: Research Funding; Aptevo: Consultancy; Merck: Research Funding; Janssen: Consultancy, Research Funding; Spectrum: Research Funding; Takeda: Research Funding; BMS: Research Funding; Seattle Genetics: Consultancy, Research Funding; Brim: Consultancy; Asana: Consultancy. Pagel:Pharmacyclics, an AbbVie Company: Consultancy; Gilead: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract