Abstract
Introduction
Multi-agent induction chemotherapy followed by autologous stem cell transplant (ASCT) is a standard of care for younger patients with multiple myeloma, aimed at maximising the depth and duration of first response (PFS1). However, the duration of PFS1 is variable between patients. Improved understanding of how to identify high risk patients who relapse early and the ability to design strategies applicable to their biology represents the central aim of personalized medicine approaches for MM. This landmarked analysis of the Myeloma XI trial was designed to identify the characteristics of patients with PFS1 of less than a year after ASCT.
Patients and methods
In MXI patients were randomised to induction therapy with CTD (cyclophosphamide, thalidomide and dexamethasone) or RCD (lenalidomide, cyclophosphamide and dexamethasone) and +/- CVD (bortezomib, cyclophosphamide, dexamethasone) intensification in patients with <VGPR to RCD or CTD. Post-ASCT patients were randomised to lenalidomide, lenalidomide+vorinostat or no maintenance treatment.
Patients who completed ASCT were identified with very early relapse (PFS1<12m) and their baseline characteristics were compared to those relapsing later (PFS1≥12m) using Chi squared test for categorical values and t test for continuous values. Differences in the PFS2 and OS were assessed and evaluated using the logrank test.
Results
Of 1274 patients receiving ASCT: 178 (14%) relapsed within 12m and 1096 (86%) thereafter. There were higher rates of ≥VGPR in the PFS1≥12m group (70% vs 59%) and in both groups most patients responded at ≥PR (PFS1<12m: 86% vs PFS1≥12m: 94%; p=0.00001). Despite this early progression was seen and 11% of the PFS1<12m group relapsed within 100d of ASCT. Fewer patients on lenalidomide maintenance showed PFS1<12m compared to observation. However, the benefit was not universal, in the PFS1<12m cohort, 37% were on lenalidomide maintenance, 13% on lenalidomide and vorinostat and 50% on observation. After first progression approximately, equal proportions of patients received second-line treatment (PFS1<12m: 55.8% vs PFS1≥12m: 54.3%), most receiving bortezomib-based combinations (80.2% vs 84.0%). Early relapsing patients were almost twice as likely to have received third-line treatment (28.8% vs 14.9%), the majority receiving lenalidomide, alone or in combinations (55.3% vs 66.7%).
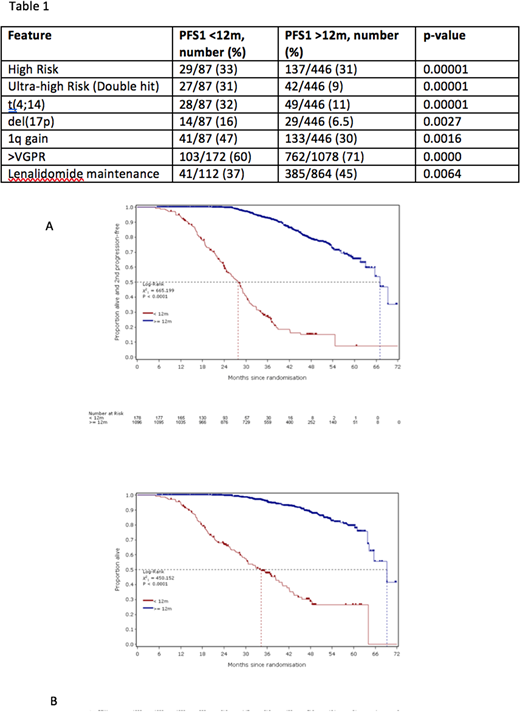
Inferior outcomes continued for the PFS1<12m group: shorter PFS2 demonstrates continued negative association with outcome beyond first-line therapy (median PFS2: 28m vs 67m; p<0.00001) (Figure 1A). This translated into significantly shorter OS for the PFS1<12m group (3-year OS: 28% vs 53%; p<0.00001) (Figure 1B).
As PFS1<12m after ASCT strongly predicts outcome, we sought characteristics of this high-risk group. There was no difference in terms of age, gender, paraprotein subtype and mean concentration or induction therapy. Notable differences included higher proportion of patients of lambda light chain subtype with PFS1<12m (43.3% vs 33.6%; p=0.0145). Mean Hb was lower for PFS1<12m: (104.6g/l (SD 19.2) vs 111.9g/l (20.1); p<0.0001) and mean plasma cell percentage was higher for PFS1<12m: (45.9% (26.9) vs 36.7% (25.3); p<0.0001). More patients had ISS stage 3 disease than ISS 1 or 2 for PFS1<12m (28.7% vs 20.3%; p=0.0068). In patients with available results, genetic analysis showed 31% of PFS1<12m patients had one high-risk lesion (HR) and 33% had two or more (UHiR), compared to 31% HR and 9% UHiR in the PFS1≥12m group. Each risk lesion was more common in the PFS1<12m group (Table 1).
Discussion
Nearly three quarters of those with PFS1<12m had died 3 years after entry to MXI, highlighting an area of urgent unmet need for this poorly understood group. Continuous lenalidomide therapy and deep serological responses did not prevent early progression in some. At 36 months post ASCT 6% of patients with PFS1<12m had not progressed for a second time compared to 33% with a PFS1≥12 months. We found 64% of patients in the short PFS group had at least one HR genetic lesion. Current standard approaches lack efficacy to salvage patients, who have been exposed to immunomodulatory drugs and proteasome inhibitors and undergo early progression, despite variation in global access to other therapies. This justifies exploration of dedicated studies to characterise these patients at baseline and develop treatments that can improve their prognosis.
Bygrave:Amgen: Honoraria; Takeda: Honoraria, Other: Travel Support; Janssen: Honoraria, Other: Travel support; Celgene: Honoraria, Other: Travel support, Research Funding. Pawlyn:Amgen: Consultancy, Honoraria, Other: Travel Support; Celgene Corporation: Consultancy, Honoraria, Other: Travel support; Takeda Oncology: Consultancy, Other: Travel support; Janssen: Honoraria, Other: Travel support. Davies:Janssen: Consultancy, Honoraria; Takeda: Consultancy, Membership on an entity's Board of Directors or advisory committees; MMRF: Honoraria; ASH: Honoraria; TRM Oncology: Honoraria; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees; Abbvie: Consultancy. Cairns:Merck Sharp and Dohme: Research Funding; Amgen: Research Funding; Celgene: Research Funding. Striha:MSD: Research Funding; Celgene: Research Funding; Abbvie: Research Funding; Janssen: Research Funding; Amgen: Research Funding. Hockaday:Millenium: Research Funding; MSD: Research Funding; Janssen: Research Funding; Celgene: Research Funding; Amgen: Research Funding; Abbvie: Research Funding. Jones:Celgene: Honoraria, Other: Travel support, Research Funding. Kishore:Celgene: Honoraria; Takeda: Honoraria, Other: travel support. Garg:Amgen: Honoraria, Other: Travel Support; Takeda: Other: Travel Grant; Novartis: Other: travel support, Research Funding; Janssen: Honoraria. Williams:Janssen: Honoraria, Other: Travel support, Speakers Bureau; Celgene: Honoraria, Other: Travel Support, Speakers Bureau; Takeda: Honoraria, Other: Travel support; Amgen: Honoraria, Speakers Bureau. Karunanithi:Janssen: Other: Travel support, Research Funding; Celgene: Other: Travel support, Research Funding. Lindsay:Celgene: Honoraria, Other: Travel support; BMS: Consultancy, Other: Travel support; Takeda: Other: Travel support; Janssen: Consultancy; Novartis: Other: Travel support. Jenner:Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; Takeda: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Chugai: Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: travel support, Research Funding, Speakers Bureau; Janssen: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Other: Travel support, Research Funding, Speakers Bureau. Cook:Amgen, Bristol-Myers Squibb, GlycoMimetics, Celgene, Janssen and Takeda and Sanofi: Honoraria; Celgene, Janssen and Takeda: Research Funding. Drayson:Abingdon Health: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Owen:Takeda: Honoraria, Other: Travel Support; Celgene: Consultancy, Honoraria, Research Funding; Janssen: Consultancy, Other: Travel support. Gregory:Janssen: Honoraria; Amgen: Research Funding; Celgene: Consultancy, Honoraria, Research Funding; Merck Sharp and Dohme: Research Funding. Morgan:Celgene: Consultancy, Honoraria, Research Funding; Takeda: Consultancy, Honoraria; BMS: Consultancy, Honoraria. Jackson:Amgen: Honoraria; Celgene: Honoraria; Chugai: Honoraria; Janssen: Honoraria; Takeda: Honoraria. Kaiser:Chugai: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Other: Travel support; Takeda: Consultancy, Other: Travel Support; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Celgene: Consultancy, Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.