Abstract
Background: The factor V Leiden mutation (FVL) and the prothrombin G20210A mutation (PRO) are the most frequent thrombophilic mutations among Caucasians. Both mutations show a highly variable expressivity, ranging from a lifelong asymptomatic course to severe venous thromboembolism (VTE). Recently, we have shown increased generation rates of thrombin and activated protein C (APC) in response to in vivo thrombin formation in asymptomatic FVL carriers (VTE-). In vivo thrombin formation was triggered by low-dose administration of recombinant activated factor VII (rFVIIa). Aim of the present study was to extend this stress test approach to carriers of PRO and to FVL carriers with a history of VTE (VTE+).
Methods: The study population consisted of 29 FVL carriers (thereof 25 heterozygotes: 13 VTE- and 12 VTE+) and 21 PRO carriers (thereof 19 heterozygotes: 12 VTE- and 7 VTE+). None of the subjects was under anticoagulant treatment at time of analysis. The control group consisted of 13 healthy volunteers tested negative for FVL and PRO. Blood samples were collected immediately before and during a period of 8 hours following injection of 15 µg/kg rFVIIa. Plasma levels of free thrombin and APC were quantified using oligonucleotide-based enzyme capture assays (OECAs). Prothrombin activation fragment 1+2 (F1+2), thrombin-antithrombin complex (TAT), and D-dimer were measured additionally.
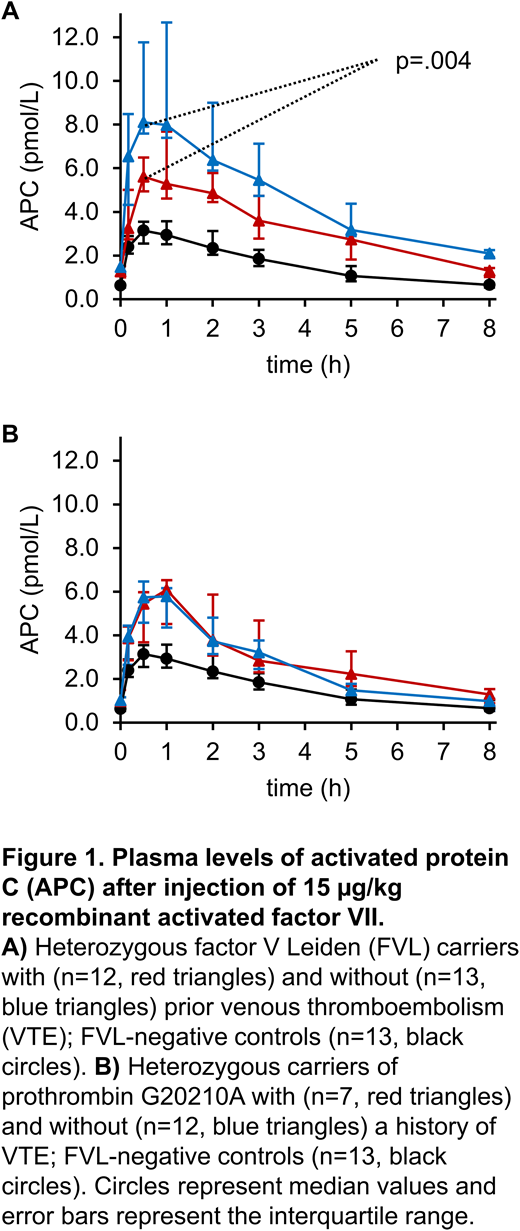
Results: Injections of rFVIIa were well-tolerated by all subjects and median D-dimer levels remained within the reference range in all groups. Compared with the controls, peak plasma levels of F1+2 and TAT were significantly higher after rFVIIa injection in FVL carriers (p=.009 and p=1.0∙10-4, respectively), and in PRO carriers (p=.045 and p=6.0·10-4, respectively), but they did not differ between the FVL and PRO cohorts, and between the VTE+ and VTE- subgroups. Median plasma concentrations of thrombin were below the limit of detection at baseline, and rFVIIa did not induce statistically significant changes of thrombin levels in any group. APC increased to peak levels 30 minutes after rFVIIa injection, from 0.63 (0.54-1.16) to 3.14 (2.55-3.55) pmol/L (p=.017) in the controls, and from 1.39 (1.14-1.86) to 7.86 (5.55-11.55) pmol/L (p<1.0∙10-4) in the FVL group. In PRO carriers, plasma levels of APC increased from 1.07 (0.86-1.23) pmol/L at baseline to a peak of 5.86 (4.44-6.41) pmol/L 1 hour after rFVIIa injection p=4.0·10-4). Peak plasma levels of APC were higher in both FVL carriers and PRO carriers in comparison to the controls (p<1.0∙10-4 and p=4.0∙10-4, respectively), and lower in PRO carriers compared with those in FVL carriers (p=.003). With peak levels of 8.11 (7.59-11.77) vs. 5.62 (4.94-6.49) pmol/L, APC was significantly higher in the VTE- subgroup than in the VTE+ subgroup (p=.004) in heterozygous FVL carriers, while in heterozygous PRO carriers, APC peak levels did not differ between the VTE- subgroup and the VTE+ subgroup (Figure 1).
Conclusion: The APC response to in vivo thrombin formation is significantly lower in symptomatic than in asymptomatic FVL carriers, implying that higher APC levels protect FVL carriers from thrombosis development. This hypothesized effect appears to be specific for FVL as prothrombin G20210A carriers do not show differences in the APC response related to VTE history. Further studies are warranted to identify the factors that modulate the APC response in FVL carriers.
Rühl:Swedish Orphan Biovitrum: Consultancy, Research Funding; Sanofi Genzyme: Research Funding; Shire: Research Funding; Grifols: Research Funding; CSL-Behring: Research Funding. Berens:Pfizer: Research Funding; Sanofi Genzyme: Research Funding; Shire: Research Funding; CSL-Behring: Research Funding; Biotest: Research Funding. Winterhagen:Swedish Orphan Biovitrum: Consultancy, Research Funding. Müller:Swedish Orphan Biovitrum: Consultancy, Research Funding. Oldenburg:Roche: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Octapharma: Consultancy, Honoraria, Research Funding; Novo Nordisk: Consultancy, Honoraria; Grifols: Consultancy, Honoraria, Research Funding; CSL-Behring: Consultancy, Honoraria, Research Funding; Chugai: Consultancy, Honoraria; Biotest: Consultancy, Honoraria; Biogen Idec: Consultancy, Honoraria; Bayer: Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria, Research Funding; Swedish Orphan Biovitrum: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.