Abstract
The epichaperome is a new cancer target defined, in part, by changes in the interaction strength between chaperone and co-chaperone proteins to form stable hyperconnected networks that support oncoprotein stability and are vital for tumor survival (Nature 2016, Nature Rev Cancer 2018 and Nature Med 2018). Cancers with this altered chaperone configuration may become susceptible to drugs that target the epichaperome, such as the inhibitor PU-H71. We have developed a novel flow cytometry-based test, the PU-FITC binding assay, to evaluate epichaperome levels at the single cell level and identify patients who are most likely to respond to PU-H71 treatment.
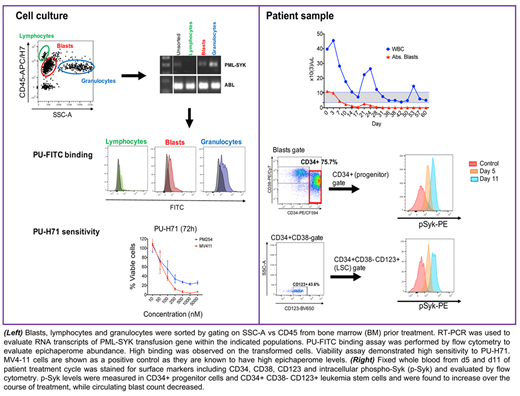
A 61-year-old woman was diagnosed with an accelerated phase myeloproliferative neoplasm in 2013 after years of leukocytosis, arthritis, urticaria, and vasculitis. Cytogenetic analysis revealed t(9;15) involving the PML gene. Molecular studies were negative for BCR-ABL, PDGFRA/B and FGFR1 rearrangements as well as JAK2 mutation. Mutations in ETV6 and multiple ASXL1 subclones were present. She was treated with hydroxyurea and in Aug 2013 underwent matched unrelated donor allogeneic stem cell transplantation conditioned with Fludarabine/Melphalan, followed by 3 cycles of vidaza in 2014 for early recurrence. In Jan 2016, she relapsed and was treated with hydroxyurea. She also had painful ulcerations of toes, thought to be an atypical presentation of graft versus host disease complicated by her underlying Raynaud's disease and treated successfully with ruxolitinib. During 2017, she had bone marrow (BM) evidence of recurrent disease, skin lesions consistent with recurrent disease, and significant splenomegaly, but since neutrophils and platelets were preserved, she did not require transfusions and she had no bleeding or infections, she was maintained on hydrea, prednisone and ruxolitinib. In 2017, BM biopsy showed progression to AML with fibrosis. She developed progressive splenomegaly and weight loss and was treated with 2 cycles of decitabine without response. The patient was sent for precision medicine evaluation. Whole-exome sequencing and RNA-seq was performed with a control buccal swab. No somatic alterations in clinically relevant genes were found. However, a novel fusion protein, PML-SYK, was detected and validated by FISH and PCR. We found constitutive Syk, Stat5, Erk and ribosomal S6 kinase phosphorylation. Elevated epichaperome levels were found in the cell populations bearing the translocation, suggesting sensitivity to PU-H71. Also, invitro treatment of patient's cells with PU-H71 resulted in cell death and decreased colony formation. These findings confirm preclinical data in AML in which a relationship between a hyperactive signalosome and epichaperome expression was observed (Cell Reports 2015). Based on the poor prognosis, lack of effective therapies, and laboratory data suggesting sensitivity to PU-H71, the patient was granted compassionate access to this medication by the FDA. After 16 doses of PU-H71 at 300 mg/m2 over 3 months, the patient has attained complete remission, with normalization of peripheral blood counts and <5% marrow blasts. Splenomegaly and all constitutional symptoms have completely resolved. Therapy is ongoing. Phosphorylation of SYK in blast as stem cell populations decreased after treatment.
In summary, a novel flow cytometry-based PU-FITC binding assay to evaluate epichaperome levels at the single cell level successfully identified a patient predicted to respond to treatment with PU-H71. This poor prognosis AML patient who had relapsed after allogeneic stem cell transplantation is presently in remission and the assay is being used to screen other potential patients.
Roboz:Sandoz: Consultancy; Janssen Pharmaceuticals: Consultancy; AbbVie: Consultancy; Eisai: Consultancy; Celgene Corporation: Consultancy; Sandoz: Consultancy; Celgene Corporation: Consultancy; Jazz Pharmaceuticals: Consultancy; Pfizer: Consultancy; Novartis: Consultancy; Orsenix: Consultancy; Aphivena Therapeutics: Consultancy; Novartis: Consultancy; Orsenix: Consultancy; Roche/Genentech: Consultancy; AbbVie: Consultancy; Argenx: Consultancy; Bayer: Consultancy; Roche/Genentech: Consultancy; Otsuka: Consultancy; Astex Pharmaceuticals: Consultancy; Otsuka: Consultancy; Argenx: Consultancy; Cellectis: Research Funding; Janssen Pharmaceuticals: Consultancy; Pfizer: Consultancy; Jazz Pharmaceuticals: Consultancy; Cellectis: Research Funding; Astex Pharmaceuticals: Consultancy; Celltrion: Consultancy; Aphivena Therapeutics: Consultancy; Celltrion: Consultancy; Daiichi Sankyo: Consultancy; Bayer: Consultancy; Eisai: Consultancy; Daiichi Sankyo: Consultancy. Morgan:Samus Therapeutics, Inc: Employment, Equity Ownership. Chiosis:Samus Therapeutics, Inc: Equity Ownership, Membership on an entity's Board of Directors or advisory committees. Guzman:Cellectis: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.