Abstract
Introduction
Residence and interaction with a specialized bone marrow microenvironment is important for normal hematopoietic stem cells and for initiation and progression of myeloid malignancies, but the role of the microenvironment in propagation and therapeutic response of acute lymphoblastic leukemia (ALL) is not well known. Prior work has identified the efficacy of inhibiting FAK signaling, which is deregulated by IKZF1 alterations resulting in induction of THY1-Integrin alpha 5 adhesion in Ph-positive (Ph+) ALL. Here, we hypothesized that this mechanism may be more broadly important in ALL. We applied a systematic integrated genomic/imaging/functional approach to define the nature of interaction and identify changes in leukemic cells upon interaction that may be targetable.
Materials and methods
Time-lapse confocal imaging was performed to examine how leukemia cells migrate and adhere to mesenchymal stem cells (MSCs). NALM6 (DUX4/ERG), MHH-CALL2 (hypodiploid), 697 (TCF3-PBX1), Reh (ETV6-RUNX1) and SUP-B15 (Ph+) cell lines were cultured with immortalized human bone marrow MSCs transduced with telomerase reverse transcriptase (hTERT) (Mihara, Br J Haematol. 2003;120:846). For RNA-sequencing, non-adherent cell line cells were collected after two days of coculture with hTERT while adherent cells were trypsinized and collected. Both samples were sorted for CD19 positive population. Fresh primary ALL samples were cultured on bone marrow MSCs derived from patients with no hematological disease and collected with the same procedure for RT-PCR. Multicolor immunofluorescence imaging was utilized to observe expression of multiple molecules involved in adherence.
Results
Time-lapse imaging showed that leukemia cells have a dynamic interaction with MSC monolayers, with temporary adherence, accompanied by dynamic change in their shape. NALM6 cells adherent to MSCs reduced cell cycling, with an increase in the ratio of G0/G1 cells (26.7% to 48.0%) and decrease in S phase (60.7 to 41.8%).
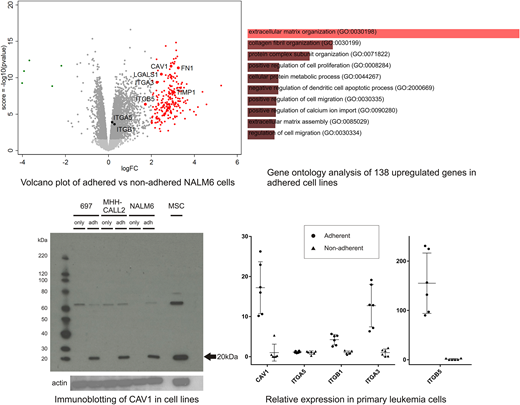
Analysis of gene expression showed 138 upregulated genes (log2FC >2 and FDR <0.05) in adherent cells which were common in all five cell lines, with striking upregulation leading to gene expression associated with gene ontology of extracellular matrix organization and collagen fibril organization. Representative genes were validated in adherent NALM6 cells by immunoblotting (FN1, TIMP1, and LGALS1).
Pathway analysis showed that transforming growth factor beta 1 (TGFB1) was the top ranked upstream regulator (p-value of overlap 5.26E-35) in that 44 of 65 genes had measurement direction consistent with activation of TGFB1 signaling. In addition, other upstream regulators that may be involved were beta-estradiol, fibroblast growth factor 2, and tumor necrosis factor.
Adhesion of leukemia cells to stroma may induce integrin expression and downstream signaling. Our transcriptomic analysis showed that integrins (A5, B1, A3 and B5) and caveolin 1 (CAV1), a main component of the caveolae plasma membranes, are highly transcribed in adherent leukemia cells. As hTERT cells showed unexpectedly low expression of THY1, a common MSC marker, we utilized primary bone marrow MSC for subsequent analysis. Immunoblotting assay showed enhanced expression of CAV1 in all cell lines adhered to MSCs. Multicolor immunofluorescence imaging demonstrated CAV1 and ITGB1 expression on leukemia cells that localized adjacent to stromal cells, which confirms that these molecules were upregulated upon adhesion to MSCs. Moreover, primary ALL cells showed remarkable upregulation of CAV1, ITGB1, ITGA3, and ITGB5 when the cells were adherent to MSCs.
Conclusions
Our results demonstrate that ALL cells dynamically interact with microenvironment cells, inducing changes in cell morphology, cell cycling, and adhesion, which may facilitate altered responsiveness to therapy. Transcriptional results suggest that TGFβ signaling is an upstream regulator after cell adhesion to MSCs. While integrins and CAV1 mediate the signaling, these pathway and molecules will be candidates for exploring inhibitors of signaling, which may affect their interaction and make them novel therapeutic targets.
Mullighan:Loxo Oncology: Research Funding; Cancer Prevention and Research Institute of Texas: Consultancy; Amgen: Honoraria, Speakers Bureau; Abbvie: Research Funding; Pfizer: Honoraria, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.