Abstract
Background: There are no FDA-approved therapies for patients with myelodysplastic syndrome (MDS) in whom hypomethylating agents (HMAs) fail, and mechanisms of resistance are not well-characterized. Preclinical and clinical data suggest that some myeloblasts express PDL-1 and that HMAs can induce expression of PD-1 on T cells, which may result in resistance through immune tolerance. We hypothesized that the addition of the PDL1-inhibitor atezolizumab to guadecitabine, a next generation HMA which has a longer in vivo exposure time, would induce or restore HMA sensitivity in patients with relapsed or refractory (R/R) MDS. Overlapping toxicities were not expected given atezolizumab is not myelosuppressive and guadecitabine does not appear to induce autoimmune or inflammatory conditions.
Methods: We are conducting a phase I/II, multicenter clinical trial for adult patients with R/R, intermediate (3+) or high-risk MDS by the revised international scoring system, including chronic myelomonocytic leukemia (CMML). A 3x3 dose escalation design for guadecitabine was used for Phase I, beginning with 30 mg/m2 (Dose level -1) days 1-5 along with a fixed dose of atezolizumab 840mg IV days 8 and 22 of a 28-day cycle. The plan was to escalate to the recommended dose of guadecitabine, 60mg/m2 (Dose Level 1) if no dose limiting toxicities (DLTs) were identified during Dose Level -1. If ≥2/6 DLTs were observed during the Dose Level 1, de-escalation to 45mg/m2 (Dose Level -1.5) would occur. The primary endpoint of phase I was safety and tolerability of the combination. Overall survival (OS) from the on-study date and overall response rates (ORR), based on the 2006 Modified IWG Response Criteria for MDS, were secondary endpoints.
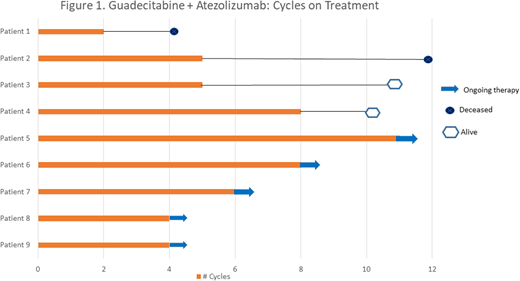
Results: Nine patients (5M, 4F, median age 73) with intermediate or higher risk were treated during phase I. Three patients were treated at Dose Level -1 and sustained no DLTs. Similarly, no DLTs were observed among 6 patients treated at Dose Level 1. There were 17 grade 3 or 4 events considered possibly or probably related to the study treatments, the most common of which were: neutropenia (4), thrombocytopenia (4), and leukopenia (4). The median number of treatment cycles was 5 and the treatment duration for each patient is illustrated in Figure 1. Two patients achieved hematologic improvement (HI) and 1 patient achieved CR. Two patients died after coming off of the study (at 4.5 and 9 months respectively) and the median OS has not been reached.
Discussion: The combination of guadecitabine at the recommended dose of 60mg/m2 D1-D5 along with atezolizumab 840mg IV d8, 22 was found to be safe with an acceptable toxicity profile in patients with R/R MDS. The ORR was 33% and a phase II study is ongoing.
Kropf:Celegene: Consultancy; Takeda: Consultancy. Grønbæk:Otsuka Pharma: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees; Janssen Pharma: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.