Abstract
Introduction
Chronic lymphocytic leukemia (CLL) is characterized by a profound immune suppression. In addition, CLL cells evade immune destruction by interacting with cells of the adaptive immune system, resulting in dysfunctional T cells. CD4+ T cells are skewed towards a TH2-profile and the number of regulatory T (Treg) cells, that diminish cellular immune responses, is increased in CLL patients. CD8+ T cells resemble exhausted T cells and have reduced cytotoxic, yet increased cytokine production capacity. The cytotoxic function of NK cells is impaired in CLL patients, but in contrast to CD8+ T cells their cytokine production is also compromised, presumably induced by CLL cells. These data are chiefly obtained from studies on peripheral blood (PB). Although the lymph node (LN) compartment has a central role in the pathobiology of CLL, very little is known about the composition of non-malignant lymphocytes in LN tissue.
The Bcl-2 inhibitor venetoclax (Ven) is highly effective in CLL and, especially in combination with anti-CD20 monoclonal antibodies such as obinutuzumab (O), results in high rates of minimal residual disease (MRD) undetectable responses. However, the prospective effects of venetoclax on non-malignant lymphocytes in patient samples remain largely unexplored.
Methods
PB and LN biopsy specimens were collected at baseline from patients enrolled in the 1st-line FCR-unfit HOVON 139 / GIVE trial. Study treatment consisted of O (cycle 1-2), Ven+O (cycle 3-8) and Ven (cycle 9-14). Immune composition was analyzed by 7-color flow cytometry. Baseline PB samples were compared to paired LN samples. Moreover, PB samples of the first patients that completed 6 cycles of Ven monotherapy (cycle 14) were compared to baseline. Cytokine production and degranulation of T and NK cells was studied after stimulation of PBMCs with PMA/Ionomycin.
Results
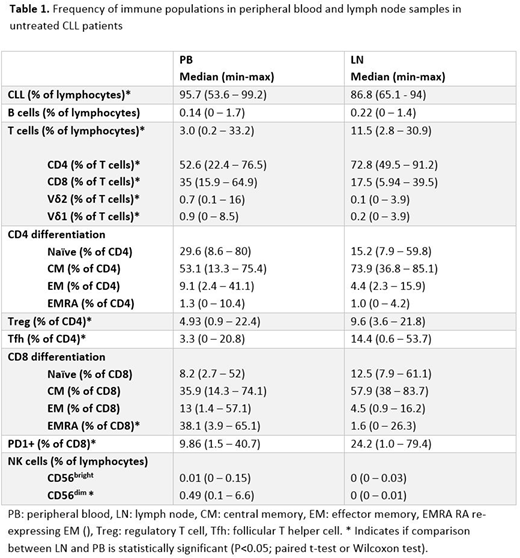
Comparison of LN (n=28) vs PB (n=48) revealed a larger proportion of T cells in LN (13.2% vs 5.1% of the lymphocytes), at the expense of CLL cells, with a skewed CD4:CD8 ratio (5.2 in LN vs 1.8 in PB). Within the CD4+ T cells, significantly higher levels of both follicular T helper cells (15. 7% vs 5.2%) and Tregs (11.5% vs 6.9%) were found in LN (see Table). CD4+ T cells mostly consisted of naïve and memory T cells in both PB and LN. There were fewer CD8+ T cells and especially fewer effector CD8+ T cells in the LN in comparison to PB. CD8+ T cells in LN mostly had a naïve and memory phenotype. An increased percentage of LN-residing CD8+ T cells expressed the exhaustion marker PD-1 as compared to PB CD8+ T cells (30.4% in LN vs 12.4% in PB).
We then compared PB baseline samples to PB obtained after cycle 14 (n=11). Ten patients achieved MRD undetectable levels (<10-4, determined by flow cytometry) and 1 patient was MRD intermediate (10-4-10-2). As expected, the treatment regimen led to complete elimination of CD19+ B cells. In contrast, absolute numbers of CD4+ and CD8+ T cells did not change during treatment. Differentiation status of CD4+ and CD8+ T cells remained similar. Interestingly, the proportion and absolute number of Tregs decreased after treatment (6.1% vs 0.9% of CD4+ T cells). After stimulation with PMA/Ionomycin, the percentage of IL-2 producing CD4+ T cells increased after treatment, leading to a higher IL-2:IL-4 ratio, that suggests normalization towards a TH1-profile.
Fewer CD8+ T cells expressed PD-1 after treatment. The fraction of CD8+ T cells that produced IFN-γ (69.8% vs 56.2%) and TNF-α (58.4% vs 40.3%) decreased. Degranulation of CD8+ T cells did not change upon treatment.
After treatment, the capacity of NK cells to degranulate increased. In addition, a larger proportion of NK cells produced IFN-γ, suggesting recovery of NK cell function after treatment.
Conclusion
In conclusion, our data strengthen the view that CLL cells reside in an immune suppressive environment in the LN. Moreover, we provide the first evidence that the Ven+O regimen does not harm non-malignant lymphocyte populations other than B cells. Both the improved cytokine production of NK cells and diminished cytokine production of CD8+ T cells may point to normalization of immune function. Collectively, the phenotypical and functional changes observed may reflect the eradication of the immunosuppressive CLL clone by Ven+O and subsequent recovery of the immune microenvironment in CLL patients.
Eldering:Celgene: Research Funding. Mobasher:F. Hoffmann-La Roche Ltd: Other: Ownership interests non-PLC; Genentech Inc: Employment. Levin:Janssen: Membership on an entity's Board of Directors or advisory committees; Celgene: Membership on an entity's Board of Directors or advisory committees. Kater:Abbvie: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Acerta: Membership on an entity's Board of Directors or advisory committees, Research Funding; Roche/Genentech: Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.