Abstract
Introduction. In the context of chemoimmunotherapy, complete remission (CR) is more common and is associated with improved survival in patients with chronic lymphocytic leukemia (CLL). CR is less frequent in CLL patients treated with ibrutinib, and the prognostic significance of achieving CR with ibrutinib is indeterminate.
Methods. We prospectively analyzed 208 CLL patients treated on a phase 2 study (NCT02007044) of first-line (deletion 17p only; n=27) or salvage ibrutinib (n=181), with or without rituximab, between 12/2013 and 01/2018. Response was assessed by international workshop on CLL 2018 guidelines. Categorical variables were compared using the χ2 or Fisher exact tests. Progression-free survival (PFS) was defined as time from treatment initiation to disease progression and/or death, and Kaplan-Meier curves compared using the log-rank test. A landmark analysis at median time of CR achievement (best response) was performed for PFS.
Results. After a median follow-up of 34 months (range, 3-48 months), response was evaluable in 194 patients, overall response rate (ORR) was 99%, and CR rate was 24%, with negative minimal residual disease (MRD) in 3% of patients; median time to response was 10 months (range, 3-45 months) and median time to CR was 21 months (5-45 months).
None of the patients' baseline characteristics associated with achievement of CR (Table).
Among the 47 patients in CR, 7 (15%) discontinued treatment, after a median time from treatment initiation of 19 months (range, 10-39); the main cause of discontinuation was toxicity (5 patients), with second cancer (metastatic melanoma) and disease progression prompting treatment discontinuation only in 2 patients. Among the 145 patients in PR, 50 (34%) discontinued treatment, after a median time from treatment initiation of 14 months (range, 4-45 months); while the main cause of discontinuation was again toxicity (26 patients), 2nd cancers and progressive disease prompted treatment discontinuation in 5 and 14 patients, respectively. Remaining causes of treatment discontinuation among patients in PR were loss to follow-up (3 patients) and consolidation therapy (2 patients).
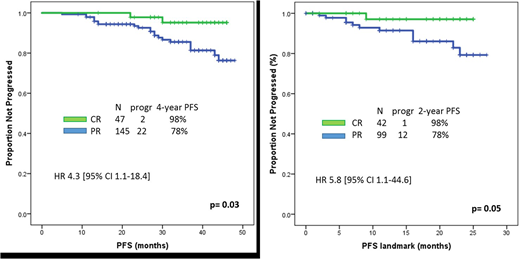
Median PFS was not reached and 28 patients (13%) progressed and/or died. Achievement of CR significantly associated with prolonged PFS (4-year PFS 98% vs 78%, p=0.03)(Figure). The association between CR and prolonged PFS was also confirmed on a landmark analysis (21 months)(p=0.05). Among baseline characteristics shown in the Table, the only factor associated with prolonged PFS was absence of complex karyotype (4-year PFS 80% vs 40%, p=0.05).
Median OS has not been reached and 16 (8%) patients have died; of these, only 1 patient was in CR (and cause of death was metastatic melanoma), whereas the remaining 15 were in PR. Among patients in PR, causes of death were: infections in 7 patients, 2nd cancers in 2 patients, Richter transformation in 2 patients and other in 4 patients (small bowel obstruction, colon perforation, intracranial hemorrhage, bradyarrhythmia).
Conclusions. This is the first study showing that achievement of CR is a desirable endpoint for patients with CLL treated with ibrutinib, associating with prolonged PFS. Our results support the development of future combination studies, aimed at achieving higher rates of CR in patients treated with ibrutinib.
Wierda:AbbVie, Inc: Research Funding; Genentech: Research Funding. Jain:Infinity: Research Funding; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Genentech: Research Funding; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Research Funding; Infinity: Research Funding; ADC Therapeutics: Research Funding; Astra Zeneca: Research Funding; Cellectis: Research Funding; Verastem: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Research Funding; ADC Therapeutics: Research Funding; BMS: Research Funding; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Research Funding; Pharmacyclics: Research Funding; Genentech: Research Funding; Abbvie: Research Funding; Celgene: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Pharmacyclics: Research Funding; Seattle Genetics: Research Funding; Seattle Genetics: Research Funding; Abbvie: Research Funding; Pfizer: Research Funding; Incyte: Research Funding; Adaptive Biotechnologioes: Research Funding; Celgene: Research Funding; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Research Funding; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Servier: Research Funding; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Research Funding; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees; ADC Therapeutics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Cellectis: Research Funding; Pfizer: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologioes: Research Funding; Servier: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novimmune: Honoraria, Membership on an entity's Board of Directors or advisory committees; Abbvie: Honoraria, Membership on an entity's Board of Directors or advisory committees; Adaptive Biotechnologies: Honoraria, Membership on an entity's Board of Directors or advisory committees; Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Astra Zeneca: Honoraria, Membership on an entity's Board of Directors or advisory committees; Verastem: Honoraria, Membership on an entity's Board of Directors or advisory committees; Novartis: Honoraria, Membership on an entity's Board of Directors or advisory committees. Thompson:Adaptive Biotechnologies: Research Funding; Genentech: Honoraria, Membership on an entity's Board of Directors or advisory committees; AbbVie: Honoraria, Research Funding; Gilead Sciences: Honoraria, Membership on an entity's Board of Directors or advisory committees; Pharmacyclics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.