Abstract
Background: Bendamustine, a bifunctional alkylator with antimetabolite activity, is an attractive combination partner for both proteasome inhibitors (PI) and immunomodulators (ImiDs) in pts. with MM. This phase I/II trial assessed the safety and efficacy of the combination of BID in pts. with RRMM exposed to bortezomib and lenalidomide and refractory to at least one of the agents.
Methods: This open label phase I/II study assessed the safety, tolerability and efficacy of BID regimen delivered in a 28-day cycle in RRMM. The primary objective of the phase I portion was to determine the recommended phase II dose (RP2D) of BID, and primary objective of the phase II portion was to estimate the overall response rates (ORR) of the combination. A 3+3 dose escalation based on dose limiting toxicities (DLTs) was employed in phase I; bendamustine was given at escalated doses of 70, 80 and 90 mg/m2 on days 1,2 of each cycle with ixazomib (4mg) and dexamethasone (40 mg) on days 1,8, 15 in a 28-day cycle for 4 cycles (up to 8 in responders). In phase II, an expansion cohort was enrolled at the RP2D using Simon 2-stage design.
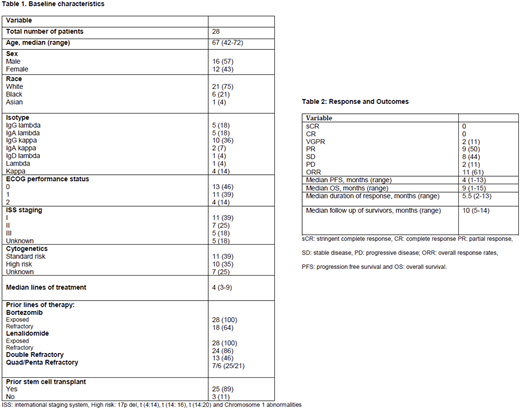
Results: A total of 28 pts. were enrolled between October 2015 January 2018. Median age of the pts. was 67 yrs. (range, 42-72), and 43% were females (Table 1). Patients received a median of 4 (range, 4-9) prior lines of therapy, of which 46% and 25% were double and quadruple refractory patients respectively and 89% had prior autologous stem cell transplant. Fifteen pts. were enrolled in phase I, and no DLTs were observed at dose level (DL) 1 and 2; while 2 /6 patients developed DLTs (neutropenia and thrombocytopenia) at DL3 establishing RP2D as bendamustine 80 mg/m2, ixazomib 4 mg and dexamethasone 40 mg. Additional 13 pts. were enrolled in phase II, and total of 19 pts. were available for phase II evaluation including pts. treated at maximal tolerated dose (MTD) in phase I (N= 6).
Of 19, 18 pts. were evaluable for response per study definition, out of which 7 completed total of 8 cycles. The median number of cycles completed was 4 (1-8). The overall response rates (ORR) was 61% with very good partial response (VGPR) 2 (11%), partial response (PR) 9(50%) and stable disease (SD) 8 (44%) and progressive disease (PD) 2 (11%). One patient completed less than 1 cycle and not evaluable for response (Table 2). For responders, the median duration of response was 5.5 months (2-13). At a median follow up of 10 months, median progression free (PFS) and overall (OS) survival were 4 months (range, 1- 13) and 9 months (range, 1-15) respectively with no treatment related morality (Table 2). The most frequent adverse events were anemia, thrombocytopenia, leukopenia, nausea, diarrhea and infections. Treatment emergent ≥ grade 3 peripheral neuropathy was not seen.
Conclusions: BID is a well-tolerated and effective combination therapy for pts. with heavily treated RRMM. Planned maintenance therapy was not used in this study and might be effective for prolonging responses.
Dhakal:Celgene: Consultancy, Honoraria; Takeda: Honoraria, Research Funding; Amgen: Honoraria. D'Souza:Celgene: Research Funding; Merck: Research Funding; Prothena: Consultancy, Research Funding; Takeda: Research Funding; Amgen: Research Funding. Hamadani:Merck: Research Funding; Sanofi Genzyme: Research Funding, Speakers Bureau; ADC Therapeutics: Research Funding; MedImmune: Consultancy, Research Funding; Ostuka: Research Funding; Celgene Corporation: Consultancy; Cellerant: Consultancy; Takeda: Research Funding; Janssen: Consultancy. Shah:Miltenyi: Other: Travel funding, Research Funding; Geron: Equity Ownership; Exelexis: Equity Ownership; Juno Pharmaceuticals: Honoraria; Oncosec: Equity Ownership; Lentigen Technology: Research Funding. Hari:Celgene: Consultancy, Honoraria, Research Funding; Amgen Inc.: Research Funding; Spectrum: Consultancy, Research Funding; Kite Pharma: Consultancy, Honoraria; Takeda: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Research Funding; Janssen: Honoraria; Sanofi: Honoraria, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.