Abstract
Introduction
Compliance with Multiple Myeloma (MM) recommendations regarding diagnosis and treatment is highly variable worldwide, outside clinical trials. This survey among hematologists from Latin America (LA) aims to describe real access to diagnostic and prognostic analyses and first line treatment for newly diagnosed MM (NDMM).
Objectives
To describe the access to diagnostic and prognostic tests and first line treatment options for MM in LA.
To compare public versus private access to tests and therapies.
Methods
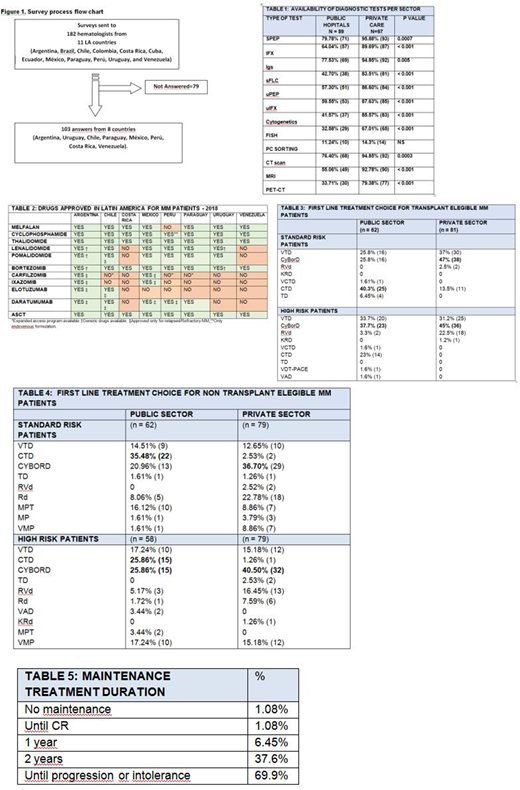
This is a multicenter cross-sectional study. A 16-question survey focusing on demographic characteristics of physicians, centers, and standard of care practices for NDMM was emailed to 182 hematologists from 11 LA countries. (Fig 1) The survey was open from Dec/17-Feb/18.
Results
We received 103 completed questionnaires (56.6%) from 8 countries: Argentina (45), Uruguay (28), Chile (15), Paraguay (6), Peru (3), Costa Rica (2), Mexico (2), Venezuela (2).
Most physicians (85/103) work in private and public institutions; the majority (64.7%) treat benign and malignant diseases, 30% mainly malignant diseases, and 4.9% mainly plasma cell disorders.
Access to diagnostic tests is shown in Table 1. In > 20% of public hospitals there is no access to serum protein electrophoresis (SPEP), serum immunofixation (IFX) or serum immunoglobulins quantification (Igs); in 57.3% serum Free Light Chain (sFLC) assay is not done. Lack of access to Fluorescent in situ hybridization (FISH) is 67%, Computed Tomography (CT) scan 23.5%, Magnetic Resonance Imaging (MRI) 45% and Positron Emission Tomography (PET/CT) 66.5%.
In private centers, lack of access to SPEP is <5%, IFX 10%, Igs 15%, sFLC 16.5%, FISH 33%, CT scan 5%; MRI 7.3% and PET/CT 20%.
Thalidomide, Bortezomib and autologous stem cell transplant (ASCT) are available in all reporting countries, in public and private institutions. Lenalidomide is reported commercially available by 97.9% of physicians, Melphalan 92.7%, Daratumumab 68%, Pomalidomide 67%, Carfilzomib 60%, Ixazomib 18%. (Table 2) Nevertheless, due to reimbursement policies not all patients have access to these drugs, as reflected in treatment choices.
In private institutions, 86.5% report treating ASCT-eligible patients upfront with Bortezomib-based triplets versus 51.6% in public hospitals [OR 5.72 (IC95% 2.32-14.7) p <0.001). Cyclophosphamide-Thalidomide-Dexamethasone (CTD) is used in the public setting by 40.3% (Table 3). Firstline choice for high risk MM (HRMM) is Bortezomib combinations in all private centers but in only 74.4% of public institutions (p<0.001).
ASCT ineligible patients: the most used upfront regimen is Cyclophosphamide-Bortezomib-Dexamethasone (CyBorD) in private centers and CTD in public hospitals (Table 4). For HRMM, Bortezomib-based triplets is the first choice in 88.57% and 65.51% in private and public settings, respectively [OR 4.09 (CI95% 1.57-11.16) p=0.0011).
Most physicians indicate maintenance treatment, mainly until progression/intolerance.(Table 5) Lenalidomide or Bortezomib are used in private centers in all countries, but for Venezuela. Only Thalidomide and Dexamethasone are available in public hospitals from 6/8 countries. Lenalidomide or Bortezomib maintenance approval require special authorization in most countries, delaying its initiation.
Discussion
This study shows real word data regarding the challenges LA faces in the care of MM patients. Access to recommended diagnostic and prognostic tests is deficient, particularly in the public setting. Diagnosis, risk assessment and response evaluation are, therefore, inaccurate. In public hospitals, >50% of patients have no access to adequate imaging evaluation, being screened by X-rays.
Regardless of commercial availability, real access to novel drugs is limited, particularly in the public setting. This causes an ethical dilemma for physicians, which must treat patients depending on the health care provider and reimbursement policies rather than based on evidence.
One limitation of this work is the high percentage of unanswered surveys.
Conclusion
LA is far from complying with international recommendations for MM approach. The most striking finding is the great difference between public and private centers in all variables. This gap is likely to translate into differences in survival, which is greatly concerning. Solving these inequities should be a priority.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.