Abstract
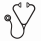
INTRODUCTION
Burkitt lymphoma (BL) is one of the most common childhood cancers across sub-Saharan Africa (Walusansa et. al, 2012). Unfortunately, the one-year survival rate of children with BL treated in low- and middle- income countries (LMICs) remains low, compared to higher-resource settings (Howard et al., 2008, Stanley et al., 2016, Buckle et al., 2016). Factors in LMICs contributing to this disparity include inability to give high-dose chemotherapy, lack of supportive care measures, and treatment abandonment (Gopal, 2018). The impact of diagnostic inaccuracies on BL outcome has not been well-studied to date.
PURPOSE
To determine the frequency and impact of an incorrect histopathologic diagnosis in children with suspected BL presenting to the Uganda Cancer Institute (UCI).
METHODS
Study Design and Participants -A sample of subjects with available tissue biopsies was selected from a cohort of children presenting to the UCI with suspected BL between July 2012 and July 2017.
Laboratory Methods - Formalin fixed, paraffin embedded (FFPE) tumor blocks were obtained from local Ugandan pathology laboratories and sectioned in a single, central Ugandan histology lab. Slides were then shipped to a US-based reference laboratory for front-line evaluation by Hematoxylin and Eosin (H&E) staining, by intentionally streamlined immunohistochemistry (IHC) for CD20, c-Myc, and TdT detection, and by EBER-1 in situ hybridization (ISH) for EBV detection. A diagnosis of BL required the expected H&E appearance and prominent tumor expression of CD20, c-Myc, and EBER-1, with no significant TdT expression. For equivocal cases, additional CD10, CD21, bcl-2, and Ki67 IHC could be employed.
Misdiagnosis Definition - A discrepancy between the pathologic diagnosis confirmed by IHC/ISH at the US-based laboratory, and the diagnosis that determined treatment in Uganda.
Clinical and Statistical Analysis - Advanced disease stage included Ziegler stage C, D, or AR based on physical exam. Kaplan-Meier and Cox regression analysis were applied to evaluate survival.
RESULTS
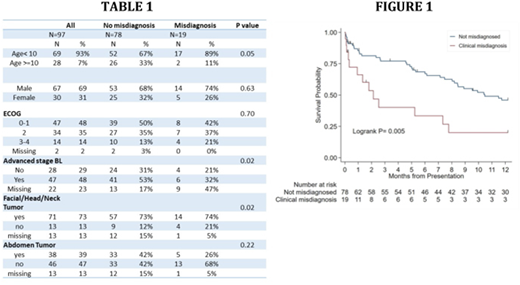
We enrolled 97 participants of with a median age of 7 (interquartile range (IQR) 4-10); 69% were male, 47% had ECOG status 0-1, and 48% had advanced stage disease (though 22% had missing staging information - Table 1). The majority of patients had facial involvement, while less than half of the evaluable patients had abdominal involvement. Twenty percent of biopsies (19/97) were misdiagnosed. Median follow-up time was 7.1 (IQR 1-12) months, during which 68% (13/19) of misdiagnosed patients died, compared to 49% (38/78) of correctly diagnosed patients. The Kaplan Meier estimate of survival among the entire cohort was 42% (95%CI 31-52%); those with and without a misdiagnosis had survivals of 20% (95% CI 5-42%) and 46% (95% CI 34-57%), respectively (Figure 1). The logrank value comparing survival among those with and without a misdiagnosis was 0.0047.
CONCLUSIONS
BL diagnosis remains challenging in resource-limited areas, with a high misdiagnosis rate of 20% in this cohort. Misdiagnosed patients tended to be younger and to have more advanced stage disease. We observed a significant positive association between misdiagnosis and early mortality. Misdiagnosis likely contributes to poorer BL survival in low-resource settings by increasing the chance of treatment for the wrong tumor type.
SIGNIFICANCE
Study limitations include relatively small sample size and the potential for selection bias among patients who had tissues samples available; however, the 12-month survival of all patients diagnosed with BL at the UCI during the study period was around 55%, and not markedly different from the 42% seen here. Next steps include a repeat study with a larger sample size. Finally, our novel IHC/ISH diagnostic algorithm, requiring 6 total slides (including 1 control slide to assess RNA quality), worked with high sensitivity and specificity, and will be described separately.
Real-Hall:Phenopath Laboratories: Employment. Adams:Burkitt Lymphoma Fund for Africa: Membership on an entity's Board of Directors or advisory committees, Research Funding. Uldrick:Celgene: Research Funding; Celgene: Patents & Royalties: 10,001,483 B2; Merck: Research Funding. Casper:Janssen: Consultancy, Research Funding; Up to Date: Patents & Royalties; TempTime: Consultancy, Other: Travel, Accommodation, Expenses; GSK: Other: Travel, Accommodation, Expenses; Roche: Consultancy, Other: Travel, Accommodation, Expenses. McGoldrick:Burkitt Lymphoma Fund for Africa: Membership on an entity's Board of Directors or advisory committees, Research Funding; Seattle Genetics: Employment. Kussick:Phenopath Laboratories: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract