Abstract
Introduction
Appropriate anticoagulation management in cancer patients is complicated by the high propensity for recurrent venous thromboembolism (VTE) as well as history of bleeding, altered anatomy, impaired organ function, nutritional issues, and intracranial tumors. The Hokusai trial demonstrated non-inferiority of edoxaban, a direct oral anticoagulant (DOAC), to low-molecular weight heparin (LMWH) in treatment of cancer-associated VTE. In the SELECT D trial, patients with cancer-associated VTE treated with rivaroxaban, another DOAC, had a 4% recurrence of VTE compared to 11% in the group treated with LMWH. These results have challenged the long standing dogma of use of LMWH for the treatment of cancer-associated VTE based on the CLOT trial.
One of the particularly challenging features of treating cancer patients with VTE is when patients have known brain tumor(s) as this heightens the concern for intracranial hemorrhage (ICH). A retrospective cohort study reported in Blood in 2015 showed no difference in ICH in patients with intracranial tumors treated with LMWH compared to matched controls not on anticoagulation. This study also confirmed that tumor histology predicts bleeding risk as the metastatic melanoma and renal cell carcinoma patients had greater risk of ICH. The risk of ICH in patients with intracranial tumors treated with DOACs remains unknown.
The aim of the present study is to compare the ICH rate in patients with intracranial tumors treated either with a DOAC or LMWH.
Methods
We performed a retrospective analysis of patients with a diagnosis of malignancy with intracranial tumor documented by imaging between May 1, 2011 and December 31, 2016. All patients were on therapeutic anticoagulation with either a DOAC or LMWH. We compared the rate of ICH in patients with intracranial tumors on treatment with DOACs to the rate in those on treatment with LMWH. Additionally, we evaluated the rate of non-intracranial bleeding and recurrent VTE in both groups. CTCAE grading was used for bleeding events. Comparisons among continuous variables were made using t-tests, and comparisons among categorical variables were made using Chi-squared and Fisher's exact tests. Associations were considered significant for P-values (two-sided) ≤ 0.05.
Results
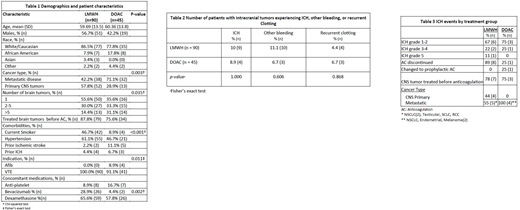
A total of 135 patients, 90 in the LMWH group and 45 in the DOAC group, were available for analysis. For patient demographics and characteristics by treatment group, see Table 1. There was a significant difference between treatment group and type of cancer, with a higher proportion of primary CNS malignancy (versus metastatic disease) in the LMWH group (71.2% vs 42.2%, p=0.003), and a higher proportion of patients in the LMWH group had only 1 brain tumor (55.6% vs 35.6%, p=0.035). There was a significant association between treatment group and whether patients were treated with bevacizumab (p=0.002), with a higher rate of bevacizumab treatment in the LMWH group (28.9% vs. 4.4%).
There was not a significant difference between treatment groups and the occurrence of ICH (10.0% LMWH vs. 8.9% DOAC, Table 2). Across treatment groups, the majority of ICH events were grade 1-2, but the LMWH group did have one grade 4 and one grade 5 ICH compared to no high grade ICH in the DOAC group (Table 3). In the LMWH group, nearly all (8/9) of the observed ICH events required anticoagulation to be discontinued. In the DOAC group, only one ICH event required anticoagulant discontinuation, whereas one other ICH event required decreased anticoagulation to prophylactic dosing. In the LMWH group, nearly all (8/9) of the observed ICH events occurred at a time when patients were not on systemic antineoplastic treatment. One ICH event in the LMWH group occurred while the patient was on therapy with a tyrosine kinase inhibitor (TKIs). In the DOAC group, two ICH events occurred at a time when patients were not on systemic antineoplastic treatment, whereas the other two events occurred while the patients were on immunotherapy. There was no significant difference between recurrent clotting events (4.4% LMWH vs. 6.7% DOAC) or other bleeding events (11.1% LMWH vs. 6.7% DOAC).
Conclusion
In patients with malignant intracranial tumors, there is no difference in the risk of ICH or other bleeding events between those on therapeutic anticoagulation with a DOAC or LMWH.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.