Abstract
The immunomodulatory drugs (IMiDs) thalidomide, lenalidomide, and pomalidomide are all approved for the treatment of multiple myeloma. IMiDs bind cereblon (CRBN), a substrate adaptor for the CRL4 E3 ubiquitin ligase (CRL4CRBN). In multiple myeloma, IMiDs enhance the binding of the lymphoid transcription factors Ikaros (IKZF1) and Aiolos (IKZF3) to CRL4CRBN, leading to their ubiquitination and degradation. Depletion of IKZF1 and IKZF3 results in growth inhibition in multiple myeloma cells. In addition, IMiDs can block the physiologic function of CRBN what has been shown to contribute to the anti-proliferative effects as well as other properties of the drug.
Recently, IMiDs were exploited for the generation of Proteolysis Targeting Chimeras (PROTACs). These molecules link the IMiD structure to another small molecule that binds a protein of interest (POI). Such IMiD-based PROTACs are capable to guide the CRBN-CRL4 E3 ligase to the POI, resulting in its ubiquitination and degradation.
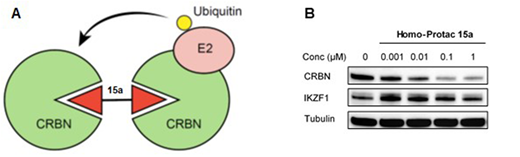
Here, we designed pomalidomide-based homobifunctional PROTACs and investigated their ability to induce self-directed CRBN ubiquitination and degradation (Figure 1A). We evaluated different attachment strategies, modifications and linker lengths and tested the effect of a series of homo-dimeric compounds on their potency to deplete CRBN protein levels. The homodimeric compound 15a with a linker length of 8 atoms was identified as the most potent CRBN degrader with a minimal remaining effect on IKZF1 (Figure 1B). Homodimeric PROTACs with longer linkers exhibited a weaker capability for CRBN degradation and had a more potent effect on IKZF1 protein levels. The effect of compound 15a on CRBN was blocked after pre-treatment with a proteasome inhibitor or MLN4924, a neddylation activating enzyem inhibitor that blocks Cullin E3 ligases. Co-immunoprecipitation revealed that 15a induces interaction of two CRBN molecules. The homo-PROTAC 15a was active at low concentrations below 100 nM and had long-lasting effects on the intracellular CRBN level (Figure 1B). Applying global proteome analyses in the multiple myeloma cell line MM1.S demonstrated that PROTAC 15a specifically induced degradation of CRBN and had only weak effects on IKZF1 and IKZF3 and no effect on the other members of the CRL4 ligase family, including DDB1 and CUL4A which are in close proximity to CRBN in the CRL4CRBN complex.
CRBN inactivation by our compounds had no effect on proliferation of different multiple myeloma cell lines. Pre-treatment with Homo-PROTAC 15a prevented pomalidomide-induced degradation of IKZF1 and IKZF3, and antagonized the effects of pomalidomide and lenalidomide on multiple myeloma cell growth. This was consistent with genetic inactivation of CRBN by CRISPR/Cas9.
In conclusion, we generated the first chemical inhibitors of CRBN that can serve as a useful tool for future biomedical investigations on CRBN-related signaling. These compounds will also help to discriminate whether an IMiD effect depends on CRBN-mediated targeted degradation of neo-substrates or from blocking the physiologic function of CRBN. Furthermore, our data confirm the essential role of CRBN in IMiD activity in multiple myeloma.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.