Abstract
Introduction
Extranodal (EN) involvement of the gastrointestinal (GI) tract in diffuse large B-cell lymphoma (DLBCL) presents heterogeneously across different anatomic sites and often occurs at initial diagnosis. Rituximab, anthracycline-based combination chemotherapy, radiation, and surgery have all been studied separately and in various combinations. However, given the variability in how patients may present in these contexts, there remains no consistent standard of care or pattern of treatment failure. We hypothesized these patients have different outcomes than those lacking this EN site of involvement.
Methods
We conducted a single-institution, retrospective analysis of consecutive cases who presented at initial diagnosis with DLBCL involving the GI tract including the esophagus, stomach, small intestine, or portion of large intestine. We performed a search in the University of Virginia Pathology database to identify all patients age >/= 18 years with DLBCL involving the GI tract and excluded those without additional clinical information available for review. A total of 51 patients were identified and we retrospectively collected data on demographics, date of diagnosis, involvement of extranodal sites, stage, International Prognostic Index (IPI) score, treatment, response to treatment, relapse, and survival at last follow-up.
Results
Of the 51 DLBCL patients, 65% of patients were male and 82% were Caucasian. Median age was 65 years (range 22-92). Three patients had HIV infection and no patients had active hepatitis B or C. Sites included gastric (51%), colon (27%), small bowel (12%), esophagus (4%), and rectum (2%); 4% had multiple GI sites involved. Forty-nine percent of patients presented with abdominal pain. Other common presentations included gastric/bowel perforation (12%), GI bleed (12%), gastric/small bowel obstruction (12%). Three cases (6%) were discovered on a screening colonoscopy. Forty-three percent of patients presented as stages I-IIIE versus 57% presented as stage IVE. Fifty-five percent were IPI 0-2 while 45% were IPI 3-5. Six patients (12%) had CNS involvement at presentation. Median follow-up was 20 months (range 26 days to 17 years). Six patients were lost to follow-up prior to assessment of response, and 4 had treatment discontinued early (3 due to grade 5 infection, 1 for GI perforation leading to death). One patient was still undergoing treatment.
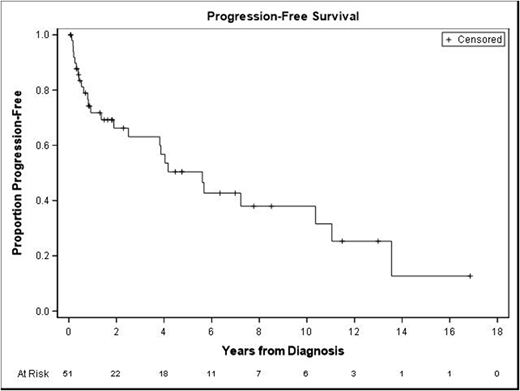
Forty patients were available to assess for response to treatment. The majority were treated with R-CHOP (57%) or DA-R-EPOCH (29%). Sixty-eight percent (n=27) had a complete response (CR), 22.5% (n=9) a partial response (PR), and 10% (n=4) were primary refractory. Of the 27 CRs, 13 (48%) had presented as stage 1-3 and 14 (52%) had presented as stage 4; 16 (59%) had an IPI score of 0-2 and 11 (41%) had an IPI score of 3-5. Of the four patients who had primary refractory disease, 3 had multiple EN sites involved at diagnosis, including 2 with CNS involvement. Of those who had a response (including CR or PR), median duration of response was 1 year (range 4 months to 13 years). Eleven patients (28%) had a relapse: 3 in the CNS, 5 in the GI tract (3 at the same site of initial involvement, 1 with a different GI site involved, and 1 with a different GI site in addition to systemic disease), and 3 in lymph nodes alone. Time to relapse ranged 1 month to 13 years with a median of 8.4 months. Fifty-seven percent of patients were alive at time of last follow-up. Median Overall survival (OS) was 5.7 years and Median Progression Free Survival (PFS) was 5.6 years (Figure 1).
Conclusion
There is limited data on how patients with DLBCL involving the GI tract initially present and respond to therapy. This study encompassing almost twenty years of experience at a single institution, suggests that though patients present at all stages and as low risk vs high risk IPI, outcomes are poor compared to prior studies, especially when additional extranodal sites are involved. Duration of response can be substantial, however relapses are common and may present years later indicating ongoing follow-up is imperative.
Portell:AbbVie: Research Funding; Amgen: Consultancy; Kite: Research Funding; TG therapeutics: Research Funding; BeiGene: Research Funding; Genentech/Roche: Consultancy, Research Funding; Acerta: Research Funding; Infinity: Research Funding. Williams:Sandoz: Consultancy; Pharmacyclics: Research Funding; Verastem: Consultancy; Seattle Genetics: Consultancy; TG Therapeutics: Consultancy; Abbvie: Consultancy; Novartis: Research Funding; Gilead: Consultancy, Research Funding; Astra-Zeneca: Consultancy; Takeda: Research Funding; Celgene: Consultancy, Research Funding; Janssen: Consultancy, Honoraria, Research Funding; Kite: Consultancy; Juno: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.