Abstract
Introduction
Dasatinib is a potent small molecule kinase inhibitor targeting BCR-ABL kinase - oncogenic driver in Philadelphia chromosome-positive (Ph+) cases of chronic myelogenous leukemia (CML). In addition to BCR-ABL kinase, it also targets a broad array of other kinases, affecting not only leukemia cells but also immune cells. Just one hour after dasatinib oral administration a rapid increase of NK, NKT, T and B cells is observed in peripheral blood. Dasatinib has been also shown to influence NK cell cytotoxicity, however, the results are discordant. Some groups observe potentiation of NK cell cytotoxic activity while others strong inhibitory effects. These inconsistencies may be explained by differences in in vitro protocols used to study this phenomenon.
Aim
Our study aims to investigate dasatinib influence on immune cells in whole blood assays resembling physiological conditions observed in patients. In particular, we want to investigate dasatinib effect on NK cell mobilization, degranulation, and anti-tumor immunity. In light of clinical and pre-clinical studies involving dasatinib and the importance of NK cells in cancer, it is crucial to establish the mechanisms and kinetics of dasatinib immunomodulatory activity.
Methods
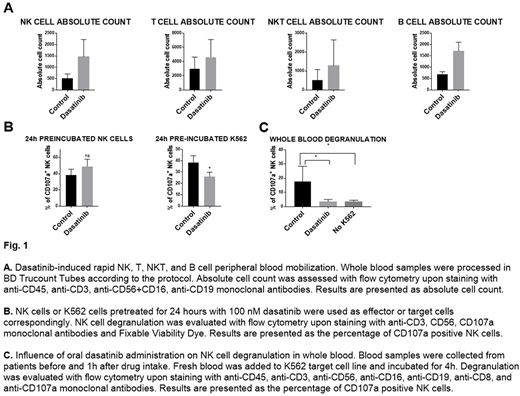
Before and one hour after first dasatinib administration peripheral blood was collected from CML patients. Collected whole blood was directly added to the target K562 cell line. After co-incubation, erythrocytes were lysed, cells were stained with a panel of monoclonal antibodies and analyzed with flow cytometry. A relative increase in lymphocyte count was determined by Trucount Tubes (BD).
Dasatinib effect on NK cells in vitro was studied with degranulation and cytotoxicity assays using NK cells isolated from healthy volunteers PBMCs. Dasatinib at clinically relevant concentrations (20-200mM) was used to assess its effect on NK cell degranulation, cytotoxicity, cytokine, and chemokine production with flow cytometry upon staining with anti-CD107a, TNF-α, IFN-γ and CCL-4 monoclonal antibodies.
For in vivo experiments C57BL/6 mice were inoculated with EL4 tumor cell line stably expressing luciferase and human CD20. 3 days after tumor inoculation mice were treated with dasatinib or vehicle intraperitoneally (i.p.) in a dose of 30 mg/kg. To monitor tumor growth, mice were injected with luciferin and imaged using the IVIS system.
Results
In agreement with previous reports, we confirm NK, NKT, T and B cell count increase in peripheral blood after dasatinib administration.
To evaluate how dasatinib influences NK cell cytokine and chemokine production we stimulated NK cells with K562 cell line. Production of major proinflammatory cytokines secreted by NK cells, TNF-α and IFN-γ, was inhibited by dasatinib treatment. Production of MIP-1β (CCL4), a chemokine secreted by NK cells attracting a broad spectrum of immune cells to inflammation sites, was also profoundly decreased.
According to our findings, dasatinib presence during the cytotoxicity assay, in a dose-dependent manner, inhibits NK cell cytotoxicity. However, 24-hour dasatinib pretreatment increases their cytotoxic potential. To better mimic the physiological conditions we used whole blood degranulation assay which closely resembles patient settings, including dasatinib concentration. One hour after dasatinib intake we observed a potent inhibitory effect of dasatinib on NK cell degranulation. Additionally, we observed a shift in NK cell subpopulations - dasatinib present during degranulation assay decreases CD16⁻ NK cell number.
Finally, we evaluated the influence of high-dose dasatinib treatment on tumor rejection in mice. Mice treated with dasatinib exhibit significantly increased tumor growth compared with vehicle-treated mice.
Conclusions
Using whole blood degranulation assay and in vitro degranulation and cytotoxicity assays we report that dasatinib effect on NK cell cytotoxicity is dose- and time-dependent. Our results indicate that dasatinib has a dual effect on NK cell degranulation and affects other NK cell functions including cytokine production and migration. Further studies are needed to evaluate the significance of these findings.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.