Abstract
OBJECTIVES: To determine whether individual items on patient-reported outcome measures show significant differences over the course of treatment for patients with cutaneous T-cell lymphoma (CTCL).
METHODS: A large, open-label, multi-center, randomized, Phase 3 study compared mogamulizumab, an anti-C-C chemokine receptor type 4 (CCR4)-targeted antibody, versus vorinostat in 372 CTCL patients who had failed ≥ 1 prior systemic therapy. Clinical quality of life (QoL) measurements included Skindex-29, Functional Assessment of Cancer Therapy-General (FACT-G), and two measures of pruritus (a Likert Scale and ItchyQoL). Analyses on identified individual symptom items of Skindex-29 and toxicity items of FACT-G were conducted using longitudinal generalized estimation equations (GEE) of the post-baseline, treated period assessments (through Cycles 5 or 6, depending on collection schedule) for these items. The proportion of patients experiencing a 1-grade improvement is presented in terms of frequency and percentage by treatment arm. Forest plots of odds ratios (OR) and associated confidence intervals (CI) from the GEE analyses were generated to characterize the likelihood of a 1-grade categorical improvement (eg, improvement by 1 category on the verbal response scale) on individual items for patients treated with mogamulizumab compared to vorinostat during the first 6 cycles of therapy.
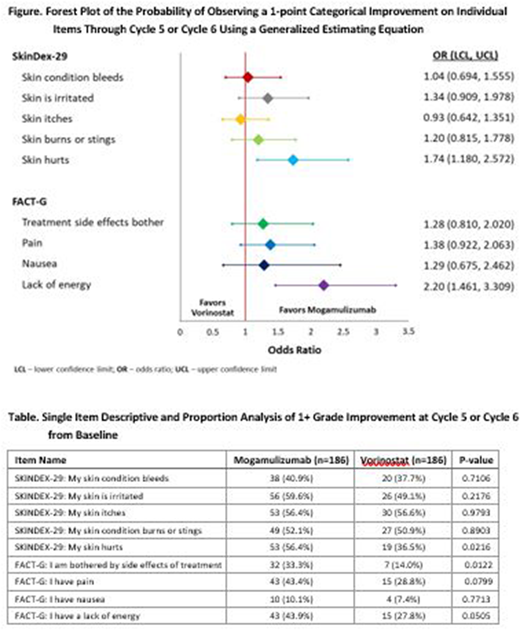
RESULTS: The likelihood of patients experiencing a 1-grade improvement in skin symptoms, side effect bother, and lack of energy was higher for patients treated with mogamulizumab compared to vorinostat (OR > 1.0). Patients treated with mogamulizumab were more likely to observe a 1-grade improvement in painful skin (OR=1.74, CI=1.180-2.572), irritated skin (OR=1.34, CI=0.909-1.978), lack of energy (OR=2.20, CI=1.461-3.309), side effect bother (OR=1.28, CI=0.810-2.020), and general cancer pain (OR=1.38, CI=0.922-2.063) within 6 cycles of therapy (Figure). The single item descriptive and proportion analysis of 1+ grade improvement at cycle 5 from baseline is presented (Table).
CONCLUSIONS: These data provide detailed information on the cumulative probability of categorical improvement of individual items on the skin symptoms of Skindex-29 and the toxicity bother, energy, and pain items of FACT-G. These results support symptom benefit of mogamulizumab over the course of treatment compared to vorinostat.
Porcu:Innate Pharma: Consultancy. Leoni:Kyowa Kirin: Employment. Duvic:Oncoceuticals: Research Funding; Precision Oncology, LLC: Membership on an entity's Board of Directors or advisory committees; MiRagen Therapeutics: Consultancy; UT MD Anderson Cancer Center: Employment; Aclaris Therapeutics Int'l Ltd.: Honoraria, Membership on an entity's Board of Directors or advisory committees; Defined Health: Consultancy; Jonathan Wood & Associates: Other: Speaker; Allos: Research Funding; Array Biopharma: Consultancy, Honoraria; Cell Medica Inc.: Consultancy, Honoraria; Concert Pharmaceuticals, Inc.: Consultancy; Guidepoint Global: Consultancy; American Council on Extracorporeal Photopheresis (ACE): Membership on an entity's Board of Directors or advisory committees; Shape: Research Funding; Medscape: Other: Speaker/Preceptor; Huya Bioscience Int'l: Consultancy; Eisai: Research Funding; Dr. Reddy's Laboratories (A.K.A. Promius Pharma): Consultancy; Forty Seven, Inc.: Membership on an entity's Board of Directors or advisory committees; Clinical Care Options: Consultancy; Huron Consulting Group: Consultancy; Millennium Pharmaceuticals, Inc.: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Soligenix, Inc.: Membership on an entity's Board of Directors or advisory committees, Research Funding; Kiniksa Pharmaceuticals: Consultancy; The Lynx Group: Consultancy; Celgene Corp: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Mallinckrddt Pharmaceuticals (formerly Therakos): Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Kyowa Hakko Kirin, Co: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; MEDACorp: Consultancy; Medivir AB: Membership on an entity's Board of Directors or advisory committees; Taiwan Liposome Company LTD: Consultancy; Evidera, Inc.: Consultancy; Rhizen Pharma: Research Funding; Spatz Foundation: Research Funding; Tetralogics: Research Funding; Seattle Genetics: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.