Abstract
Introduction
A clinical indication for allogeneic hematopoietic stem cell transplantation (HSCT) in adult Philadelphia-chromosome negative [Ph (-)] acute lymphoblastic leukemia (ALL) patients in complete remission 1 (CR1) remains to be clarified. Minimal residual disease (MRD) status has been reported to be a strong prognostic factor in adult ALL patients (Blood. 2006 107:1116, 2009 113:4153)( J Hematol Oncol. 2013 6:14). We prospectively monitored MRD status during induction and consolidation therapy in adult Ph (-) ALL patients to determine a clinical indication for HSCT.
Materials & Methods
From December 2008 to November 2013, 103 adult ALL patients were enrolled in this study. Eligibility criteria included non-L3 ALL, 16-65 years of age, and adequate organ function. Of these patients, 95 were eligible for this study and 59 were Ph (-). The treatment protocol used in this study was modified CALGB 19802. Treatment consisted of 6 courses given in the order of A-B-C-A-B-C, followed by a maintenance phase. Induction chemotherapy (course A) consisted of cyclophosphamide (1,200 mg/m2), daunorubicin (60 mg/m2 x 3), vincristine (VCR) (1.3 mg/m2 (max 2mg) x 4), L-asparaginase (3,000 U/m2 x 6) and prednisolone. Granulocyte colony-stimulating factor was started on day 4 and continued until neutrophil recovery. Consolidation B consisted of mitoxantrone (10 mg/m2 x 2), cytarabine (2,000 mg/m2/day x 4) and intrathecal (IT) administration of methotrexate (MTX). Consolidation C consisted of VCR (1.3 mg/m2 (max 2mg) x 3) and MTX (1,500 mg/m2 x 3) with leucovorin rescue and IT MTX. Patients received maintenance chemotherapy on a monthly basis: prednisolone 60 mg/m2 on days 1-5, VCR (1.3mg/m2 (max 2mg) x 3) on day 1, oral MTX 20 mg/m2 weekly, and oral 6-mercaptopurine 60 mg/m2 daily. MRD status was evaluated after induction therapy (first course A) and after second consolidation therapy (first course C). When MRD statuses after the consolidation were positive, patients were supposed to proceed to HSCT whenever possible.
Results
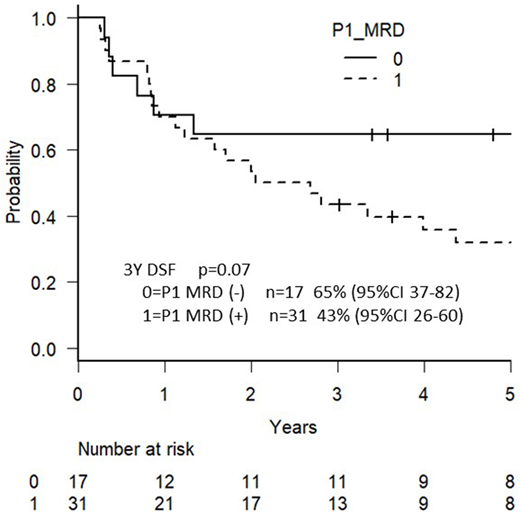
Among the 59 Ph-negative ALL patients, 51 patients (86%) could be monitored for MRD status, and the remaining 8 patients were not because of no clonal TCR/Ig targets or chimeric mRNA. The MRD status was determined by PCR analysis of major gene rearrangements and/or chimeric mRNAs (18 were positive for TCRγ, 16 for TCRδ, 13 for IgH, 1 for TCRγ and MLL-AF4, 1 for TCRγ and ETV6-RUNX1, 1 for IgH and MLL-AF4, and 1 for IgH and MLL-ENL). MRD quantifications were performed using ASO-primers with a sensitivity of ≤1 × 10−4, and MRD positivity was defined as a lower limit of detection of ≥1 × 10−3. The median follow-up time was 1597 days (range, 16-2870 days). A total of 51 patients included 29 males and 22 females, and their median age was 29 years ranging from 16 to 65. The median white blood cell count at presentation was 8.5 × 103/L (range 1.2-650.4). CR was achieved in 45 patients (88%). One patient died during induction due to intestinal bleeding. There were 29 survivors after the median follow-up period. The probability of 3-year OS and DFS in these patients with Ph-negative ALL was 69% (95%CI 54-80) and 50% (95%CI 36-63, respectively. In terms of CR1 status, MRD-negative patients after induction chemotherapy A in the first course (n = 17) showed a better 3-year DFS (65%) compared with MRD-positive patients (n = 31; 43%), as shown in Figure 1. The difference was not statistically significant (p = 0.07). There was no patient who proceeded to allogeneic HSCT among 17 MRD-negative patients after induction therapy in CR. In contrast, patients who were MRD-positive after induction but became MRD-negative after consolidation chemotherapy C in the first course (n = 11) showed a worse 3-year DFS compared with patients who were MRD-negative after induction chemotherapy A in the first course (45% vs. 65%, p = 0.08). Fourteen patients were MRD-positive after consolidation chemotherapy C in the first course. Among them, 5 patients proceeded to allogeneic HSCT in 1CR, and 9 did not. Three-year DFS with or without allogeneic HSCT were 60% (95%CI 13-88) vs 44% (95%CI 14-72, p=0.52), respectively.
Discussion
The present study indicates that MRD status after induction and consolidation therapies is a strong prognostic factor. Post-induction MRD-negative patients have been identified to have good-prognosis with chemotherapies, not suitable for up-front allogeneic HSCT.
Takamatsu:Taisho Toyama Pharmaceutical: Research Funding; TAIHO Pharmaceutical: Research Funding; Pfizer: Research Funding; Bristol-Myers Squibb: Research Funding; Ono Pharmaceutical: Research Funding; Astellas Pharma: Research Funding; Kyowa Hakko Kirin: Research Funding; Chugai Pharma: Research Funding; Takeda Pharmaceutical: Research Funding; Celgene: Honoraria. Akashi:Celgene: Research Funding, Speakers Bureau; Novartis pharma: Research Funding; Ono Pharmaceutical: Research Funding; Eisai: Research Funding; sanofi: Research Funding; Pfizer: Research Funding; Chugai Pharma: Research Funding; Kyowa Hakko Kirin: Research Funding, Speakers Bureau; Asahi-kasei: Research Funding; MSD: Research Funding; Otsuka Pharmaceutical: Research Funding; Taiho Pharmaceutical: Research Funding; Bristol-Myers Squibb: Research Funding, Speakers Bureau; Astellas Pharma: Research Funding; Eli Lilly Japan: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.