Abstract
Introduction: Chimeric antigen receptor (CAR)-modified T cells targeting CD19 can induce potent and sustained responses in children with relapsed/refractory acute lymphoblastic leukemia (rrALL). We previously reported a robust intent-to-treat product-manufacturing success rate of 100% in minimally selected, heavily pretreated patients with ALL in our Pediatric and young adult Leukemia Adoptive Therapy (PLAT)-02 phase 1 study (NCT02028455). Following completion of the Phase 1 study, a minimal change in CAR T cell manufacturing (henceforth referred to as SCRI-CAR19v2) was introduced with product infusion in 21 subjects.We sought to compare efficacy and toxicity of the subjects who received SCRI-CAR19v2 with those subjects enrolled on the Phase 1 portion of the trial (Gardner et al., Blood 2017).
Methods: All subjects underwent apheresis, and CD4 and CD8 T cell subsets are selected immunomagnetically. SCRI-CAR19v2 manufacturing changes from the previously published phase 1 platform included no longer selecting cells in culture based of EGFRt expression and changing the manufacturer of the anti-CD3/CD28 bead stimulation. All patients received fludarabine/cyclophosphamide lymphodepletion (LD) followed by bedside thaw of CD4 and CD8 T cell products and infusion of each product at a 1:1 ratio for a total of 1x106 CAR-T cells/kg. Cytokine release syndrome (CRS) was graded according to Lee et al. and treated with our early intervention strategy of tocilizumab and dexamethasone for persistent, mild CRS.
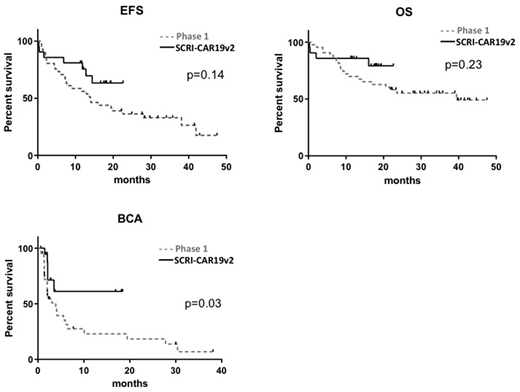
Results: Twenty-one subjects with CD19+ rrALL received SCRI-CAR19v2, with a median age of 13 years (range, 8-17 years), 10 subjects (47.6%) had a history of at least 1 prior allogeneic transplantation, with a range of 335 days from most recent transplantation before enrollment. The disease burden included 7 (33.3%) subjects having M3 bone marrow (BM), 5 (24%) having active CNS involvement at time of LD. Thirteen (62%) subjects had a high antigen burden (>15% CD19 antigen expressing cells in BM by flowcytometry prior to LD). CAR T cell products were released on all 21 subjects. 86% (18/21) of subjects had a documented minimal residual disease (MRD)-negative complete remission (CR) within 21 days following CAR-T cell therapy. The duration of functional CAR-T cell persistence, as measured by ongoing B-cell aplasia (BCA) was 61.1% at 1 year, compared to 20.6% for Phase 1 subjects (p=0.03). With a minimum of 1-year follow up (range 12-18 months), the 12-month event-free survival (EFS) and overall survival were 76% (95% CI 59-97) and 86% (95% CI 72-100), respectively; compared to 50.8% (95% CI, 36.9%-69.9%) and 69.5% (95%CI, 55.8%-86.5%) of phase 1(p=0.14 and 0.23, Figure 1). Of the 5 leukemic relapses, 1 lacked CD19 and 2 recurred as AML. Any grade CRS was seen in 71% (15/21) of infused subjects, with a maximum grade of 2. Although the grading scale from phase 1 to 2 was changed from CTCAE to Lee et al, there did not appear to be any differences in the rate of those requiring low dose vasopressors. Any grade neurotoxicity was seen in 67% (14/21) with grade ≥3 in 24% (5/21), which was similar to the phase 1, however, there was a single event of grade 5 cerebral edema, which had not previously been seen. Comparison of SCRI-CAR19v2 phenotype and functional attributes to SCRI-CAR19v1 revealed evidence of increased CD4 differentiation in the SCRI-CAR19v2 products (lower expression of CCR7, CD27, CD127, high expression of LAG-3 and TIM-3) while SCRI-CAR19v1 products showed a lower frequency of LAG-3, PD-1 and the percentage of cells secreting IFNg, suggesting a less activated phenotype.
Conclusions:A small manufacturing change was associated with a more effector driven phenotype of the SCRI-CAR19v2 T cell product, which appears to result in longer CAR-T cell persistence (prolonged BCA). Although the overall rates of CRS and neurotoxicity appeared similar between the two groups, the single event of fatal cerebral edema invoked concern that it could be related to the more effector driven phenotype. Much of the discussion surrounding the toxicity profiles and long term persistence of CAR T cell products has focused on the construct itself, including the FcFv and co-stimulatory molecules. However here we show that a change in the manufacturing platform itself can have an impact on the in vivo performance of the CAR T cell product, potentially effecting both the toxicity profile and the ability to enhance long term persistance.
Pulsipher:Amgen: Honoraria; CSL Behring: Consultancy; Novartis: Consultancy, Honoraria, Speakers Bureau; Adaptive Biotech: Consultancy, Research Funding. Park:Bristol-Myers Squibb: Membership on an entity's Board of Directors or advisory committees. Jensen:Juno Therapeutics, Inc.: Consultancy, Patents & Royalties, Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.