Abstract
Background
The Philadelphia chromosome−negative classical MPNs (myelofibrosis [MF], polycythemia vera [PV], and essential thrombocythemia [ET]) are associated with a pronounced symptom burden, including fatigue, night sweats, itching, and inactivity that may affect patients' HRQOL. There is a limited real-world evidence from many of the Asian countries, including Middle East, regarding the impact of MPNs on HRQOL, or the economic burden associated with these diseases. MERGE is a multinational, multicenter, nonintervention study conducted in adult patients with MPNs in Asia, including Middle East, Turkey, and Algeria. The present analysis evaluated the impact of MPNs on HRQOL of patients from MERGE and explored the association of HRQOL with the clinical outcomes and resource utilization.
Method
The MERGE registry included patients with MPN diagnosed as per the World Health Organization 2008 criteria. Patients were planned to be followed up for 2 years (5 visits over the course of study). MPN Symptom Assessment Form Total Symptom Score (MPNSAF TSS) was administered during the routine biannual follow-up visits to assess the HRQOL. MPNSAF TSS score was calculated only for patients who completed at least 6 of the 10 items on the MPNSAF TSS questionnaire. The level of inter-rater agreement between physicians and patients on the proportion of symptomatic patients was assessed through Cohen's Kappa coefficient.
Further, an ANCOVA (Analysis of covariance) model was applied to estimate the influence of the baseline variables (age, sex, weight, height, family history of MPNs, comorbidities, time to initial MPNs diagnosis, type of MPN initial diagnosis, baseline hemoglobin and platelet count, Eastern Cooperative Oncology Group Performance Status [ECOG PS], and time since diagnosis to treatment initiation) on the TSS score. Variables with univariate P-value < 0.20 were included in the multivariate model.
Furthermore, medical resource utilization was assessed by evaluating the inpatient and outpatient visits, hospice care, emergency room visits, and day care.
Results
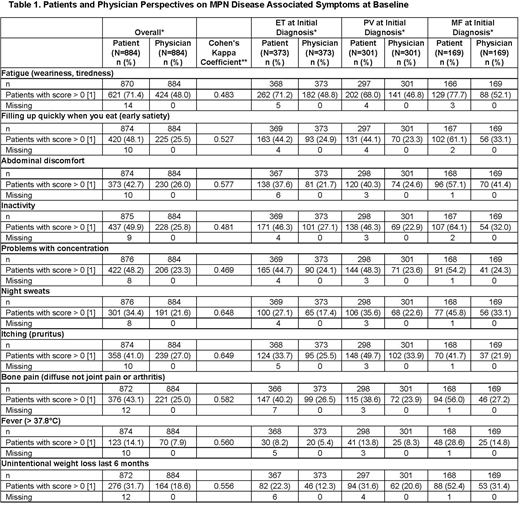
Of the 884 eligible patients with MPNs, 169 had MF, 301 had PV, 373 had ET, and 41 had unclassified MPN. During the study, 64% of patients received hydroxyurea, 15% interferon, 15% JAK inhibitors, and 10% anagrelide either alone or in combination. At baseline, the proportion of patients presenting at least 1 of the symptoms was higher in the MF group (96.4%), compared to the total MPN patients (91.8%). In addition, the TSS (mean [SD]) at baseline was highest in the patients with MF (23.5 [17.47]) as compared to patients with ET (14.6 [14.26]) and PV (16.6 [14.84]). During the study duration and follow-up, the TSS remained approximately constant at subsequent visits.
Physicians reported a lower proportion of symptomatic patients than the proportion self-reported by patients, although, there was better agreement between physicians and patients for pruritus and night sweats (Kappa, 0.61-0.80) (Table 1) than for other symptoms (Kappa, 0.41-0.60). Across MPN subtypes, inter-rater agreement was lowest for patients with MF compared to patients with ET or PV.
In a multivariate adjusted mixed effect regression model, female gender, type of MPN (MF), presence of comorbidities, lower baseline hemoglobin were independently and positively associated with the symptom score values.
Overall, 24.3% of the patients had at least 1 inpatient visit, with a mean duration of 11.1 days. Outpatient visits were the most commonly observed healthcare resource used (95.5%), without remarkable change during the follow-up period. Patients with ET had a lower mean number of inpatient visits (mean [SD]: 0.9 [0.77] days), and patients with MF had more outpatient visits (mean [SD]: 5.2 [3.17] visit) on an average, compared to the entire MPN group.
Conclusion
Despite being on treatment, patients with MPN in MERGE had substantial symptom burden, which did not change over the course of the study. A discordance between physician and patient perception of symptom assessment was seen in this study, indicating that in addition to spleen and hematological investigations, a more systematic assessment such as the use of MPNSAF TSS could help to better evaluate patients' symptoms and understand the disease and its treatment burden.
Taher:Celgene Corp.: Research Funding; Ionis Pharmaceuticals: Consultancy; Protagonist Therapeutics: Consultancy; La Jolla Pharmaceutical: Research Funding; Novartis: Consultancy, Honoraria, Research Funding. Yassin:Novartis: Research Funding. Rippin:Employee of IQVIA - doing consultancy for Novartis: Consultancy. Sadek:Novartis Pharmaceutical Corporation: Employment. Siddiqui:Novartis Pharma AG: Employment, Equity Ownership. Wong:Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.