Abstract
Introduction: Idelalisib is a first-in-class PI3Kδ-inhibitor. In clinical studies idelalisib demonstrated significant efficacy in patients with CLL, including patients with TP53 aberrations (del17p and/or TP53m). On this basis national and international guidelines in Europe recommend idelalisib as one treatment option in this high-risk CLL patient population. However, it is unclear how efficacy reported in clinical studies translates into real world experience. Considering the importance of such data, we initiated a real world study soon after market authorization of idelalisib in the European Union prospectively investigating efficacy and safety of idelalisib in routine clinical practice. Concomitant PJP prophylaxis is a risk minimization measure that was introduced after market authorization of idelalisib. Nevertheless, the impact on patient outcomes in routine clinical practice has not been studied in detail. We therefore also analyzed the impact of PJP prophylaxis on overall survival (OS) within this real world cohort.
Methods: A prospective, two-cohort, multicenter, non-interventional post-authorization safety study (PASS) reporting real world safety and efficacy data on the use of idelalisib in Germany. Inclusion of patients was based on the physician's decision to initiate treatment with idelalisib in accordance with the European Summary of Product Characteristics. Descriptive statistics were used for data analysis.
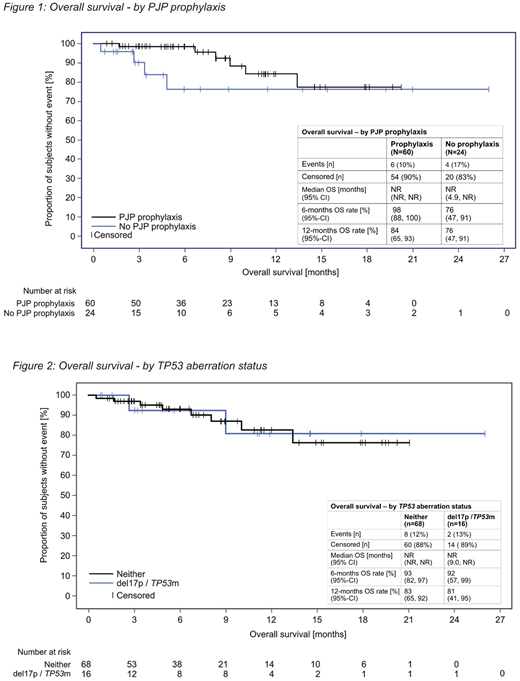
Results: This analysis included 84 CLL patients with a median age of 74 years. 88% of patients were older than 65 years, 70% were male and 86% presented with one or more co-morbidities. Binet stage A, B, C was reported in 25%, 33% and 37% of patients, respectively. The median time from diagnosis to start of idelalisib therapy was 89.5 months and patients received a median number of two prior lines of therapy, including treatment with the BTK inhibitor ibrutinib in 11 patients (13%). With a median observation time of 11.5 months the median overall survival (OS) for the entire CLL patient population was not reached. Our CLL cohort included 24 patients (29%) that did not receive PJP prophylaxis for idelalisib therapy. We therefore compared OS in patients with and without concomitant PJP prophylaxis. In patients receiving PJP prophylaxis survival rates were higher in the first 6-12 months of therapy, with 6-month and 12-month survival rates in patients with vs without PJP prophylaxis of 98% vs 76% and 84% vs 76%, respectively (Figure 1). 19% of CLL patients (n=16) had documented TP53 aberrations, including five patients that received idelalisib as first-line treatment. In patients with TP53 aberrations the overall response rate (ORR) was 77% compared to 67% in patients without TP53 aberrations. Importantly, the 12-month survival rates for patients with and without TP53 aberrations were similar with 81% and 83%, respectively. Median OS was not reached for either patient population (Figure 2).
Conclusion: This prospective real world study started collecting data on the efficacy and safety of idelalisib in routine clinical practice soon after market authorization of idelalisib in Europe. Results demonstrate similar efficacy of idelalisib irrespective of the patients' TP53 status confirming the efficacy previously reported in pivotal clinical studies. Additionally, our results provide evidence that PJP prophylaxis is an effective risk minimization measure impacting on survival.
Hoechstetter:Hexal: Other: Travel Grants; Abbvie: Other: Travel Grants; Gilead Sciences: Consultancy, Other: Travel Grants. Eissmann:Gilead Sciences: Employment. Hucke:Gilead Sciences: Employment. van Troostenburg:Gilead Sciences: Employment. Ramroth:Gilead Sciences: Employment. Knauf:Celgene: Consultancy, Honoraria; Gilead Sciences: Consultancy; Janssen: Consultancy; Mundipharma: Consultancy; Roche: Consultancy; Amgen: Consultancy, Honoraria; AbbVie: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.