In this issue of Blood, Kusumoto et al report that patients with resolved hepatitis B (hepatitis B surface antigen [HBsAg] negative, anti-hepatitis B core antibody [anti-HBcAb] positive) receiving obinutuzumab or rituximab-based treatment on the GOYA and GALLIUM studies experienced a substantial risk of hepatitis B virus (HBV) reactivation (10.8% if not administered prophylaxis).1
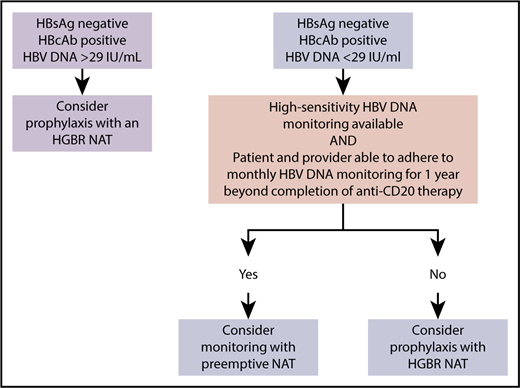
An approach to resolved HBV in patients requiring anti-CD20 therapy for lymphoma that prioritizes safety and feasibility. HGBR, high genetic barrier to resistance.
An approach to resolved HBV in patients requiring anti-CD20 therapy for lymphoma that prioritizes safety and feasibility. HGBR, high genetic barrier to resistance.
It has long been recognized that patients with chronic HBV (HBsAg positive) experience a high risk of HBV reactivation when exposed to rituximab.2 More recently, it has been recognized that patients with so-called resolved infection also have a risk of HBV reactivation with rituximab.3 Kusumoto et al’s report is the first to demonstrate that obinutuzumab is associated with an important risk of HBV reactivation in this population as well.
Kusomoto et al report that prophylactic nucleos(t)ide analog therapy (NAT) is strongly associated with a reduced risk of HBV reactivation; however, despite this, they argue that prophylactic NAT may not be necessary in all patients with resolved HBV.1 In GOYA and GALLIUM, patients were prospectively screened for HBsAg and anti-HBcAb. Investigators had the option to treat patients with resolved HBV with a prophylactic NAT of their choice or monitor HBV DNA levels monthly for 1 year beyond completion of anti-CD20 treatment with preemptive (triggered) NAT given if HBV DNA levels rose above 29 IU/mL, a very low threshold definition of HBV reactivation. Patients with quantifiable HBV DNA (>29 IU/mL) at baseline were not eligible for this study. Importantly, Kusumoto et al report that while more HBV reactivations occurred with the preemptive/triggered strategy, there were no episodes of HBV-hepatitis in either arm, suggesting that both strategies were safe.
The authors argue that avoiding prophylactic NAT may be desirable due to the cost of prolonged treatment, potential side effects from NAT, a risk of HBV resistance, and the risk of rebound HBV reactivation when antiviral medications are stopped. NATs are generally well tolerated with infrequent side effects. Viral resistance, however, is a well-recognized and potentially dangerous phenomenon when low-resistance-barrier NATs such as lamivudine are used.4 Fortunately, HBV resistance is uncommon with high-resistance-barrier NATs such as entecavir and tenofovir5,6 and has not been reported in the context of preventing HBV reactivation in cancer patients. Rebound HBV reactivation has been reported following discontinuation of prophylactic NAT,7 but the risk is unknown and, in most cases, can be mitigated by testing HBV DNA levels after stopping a NAT. Thus, the relative risks and benefits of HBV prophylaxis vs a preemptive strategy are not clear.
The Kusomoto et al paper suggests that in the context of a clinical trial, monitoring for HBV reactivation with preemptive NAT is a safe strategy, particularly for patients with undetectable HBV DNA at baseline and measurable HBsAb.1 Whether or not this approach can or should be generalized to the real world is less clear and will likely depend on a combination of feasibility and economics.
The HBV monitoring strategy employed by Kusomoto et al (monthly HBV DNA testing for 1 year beyond last anti-CD20 dose) was appropriately conservative and thus also resource intensive. Many regions do not have ready access to HBV DNA testing8 or have access to a less sensitive HBV DNA assay then used in the reference study. Kusomoto et al’s results should not be extended to such centers. Even in regions with access to sensitive HBV DNA testing, it is important for clinicians to be realistic regarding their patients’ and clinics’ ability to adhere to monthly HBV DNA testing for a prolonged period of time. A large body of evidence documents ongoing challenges achieving high screening rates for HBV prior to cancer treatment.9 In this environment, one wonders if monthly HBV DNA testing is feasible, particularly given that after completing lymphoma treatment, most patients transition to less frequent contact with their oncology provider. Indeed, even in the context of the clinical trial, Kusomoto et al report 2 patients experienced high HBV DNA levels after delays in their scheduled monthly HBV DNA tests.1
Economics will also impact the ideal real-world management strategy for patients with resolved HBV receiving an anti-CD20 antibody. In many regions, patients bear the cost of not only drugs but also monitoring tests, necessitating difficult tradeoffs for patients and their providers. Generic high-barrier NATs are now available in most regions; however, the cost of generic drugs can vary dramatically between jurisdictions. Similarly, both the accessibility and cost of HBV DNA monitoring varies. In some regions, it may be less costly to provide prophylactic high-barrier NAT with less frequent HBV DNA monitoring than to monitor HBV DNA monthly for years.
As with all important studies, the Kusomoto et al paper answers some questions but raises others. Monthly HBV monitoring appears to be safe for most patients with resolved HBV receiving anti-CD20 therapy (provided testing is adhered to), but it is unknown whether less frequent, and thus less costly, HBV DNA monitoring might be adequate. Similarly, it is not known whether monitoring for reverse seroconversion (converting from HBsAg negative to positive) might be sufficient. New HBsAg immunoassays with sensitivities approaching that of polymerase chain reaction are in development; as these become more widely available, they may become the monitoring assay of choice. Other new tools such as HBV RNA and HBV core-related antigen correlate with intrahepatic HBV DNA transcription and may allow for better prediction of the risk of reactivation. Finally, as mentioned above, many decisions in this realm are influenced as much by economics as by clinical trials. Formal cost-effectiveness analyses comparing different HBV management strategies are needed; however, the results will almost certainly vary by region.
In the meantime, what is the clinician faced with a patient with resolved HBV and lymphoma to do? We outline one approach (see figure) that is based in part on the results of the Kusomoto et al paper, previous literature,10 and our belief that for any monitoring strategy to be safe, anticipated adherence to monitoring needs to be high.
Conflict-of-interest disclosure: L.K.H. has received research funding from Gilead. J.J.F. has received research funding from Abbvie, Gilead, Janssen, and Merck and has completed consultancy work for Abbvie, Contravir, Enanta, Gilead, and Merck.