Key Points
Outcomes after RT for stage I and localized stage II FL after PET-CT staging are better than those in historical series.
More than two-thirds of patients remain in remission at 5 years, and most relapses occur at distant sites.
Abstract
Radiotherapy (RT) can be curative in patients with localized follicular lymphoma (FL), with historical series showing a 10-year disease-free survival of 40 to 50%. As 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography with computerized tomography (PET-CT) upstages 10 to 60% of patients compared to CT, we sought to evaluate outcomes in patients staged by PET-CT, to determine if more accurate staging leads to better patient selection and results. We conducted a multicenter retrospective study under the direction of the International Lymphoma Radiation Oncology Group (ILROG). Inclusion criteria were: RT alone for untreated stage I to II FL (grade 1-3A) with dose equivalent ≥24 Gy, staged by PET-CT, age ≥18 years, and follow-up ≥3 months. End points were freedom from progression (FFP), local control, and overall survival (OS). A total of 512 patients treated between 2000 and 2017 at 16 centers were eligible for analysis; median age was 58 years (range, 20-90); 410 patients (80.1%) had stage I disease; median RT dose was 30 Gy (24-52); and median follow-up was 52 months (3.2-174.6). Five-year FFP and OS were 68.9% and 96%. For stage I, FFP was 74.1% vs 49.1% for stage II (P < .0001). Eight patients relapsed in-field (1.6%). Four had marginal recurrences (0.8%) resulting in local control rate of 97.6%. On multivariable analysis, stage II (hazard ratio [HR], 2.11; 95% confidence interval [CI], 1.44-3.10) and BCL2 expression (HR, 1.62; 95% CI, 1.07-2.47) were significantly associated with less favorable FFP. Outcome after RT in PET-CT staged patients appears to be better than in earlier series, particularly in stage I disease, suggesting that the curative potential of RT for truly localized FL has been underestimated.
Introduction
Follicular lymphoma (FL) is the most common form of indolent non-Hodgkin lymphoma (NHL). Patients typically present with advanced-stage disease and are generally considered incurable, although with modern chemoimmunotherapy, median overall survival (OS) for this patient group is now approaching 15 to 20 years.1,2
For the minority with localized stage I or II disease, definitive radiotherapy (RT) can be curative, with historical series reporting 10-year disease-free survival rates of 40% to 50%, with few relapses seen beyond this time.3,4
18F-fluorodeoxyglucose (18F-FDG) positron emission tomography with computerized tomography (PET-CT) is now considered the gold-standard imaging technique for staging FL.5,6 More than 95% of FLs are FDG avid.7-9 Upstaging occurs in 10% to 60% of cases compared with conventional CT staging alone.10-13
FL is a highly radiosensitive lymphoma. In-field relapse after RT is rare, with most relapses occurring distantly.14,15 This demonstrates that in many cases, recurrence is not due to failure of RT but instead results from the presence of occult disease outside of the radiation fields.
The aim of this study was to evaluate outcomes of patients staged with PET-CT and treated with RT alone for stage I/II FL. Our hypothesis was that with more accurate staging, patients would be better selected for treatment, with consequent improvement in treatment results.
Methods
Patients
We conducted a multiinstitutional retrospective study including 16 international centers, under the direction of the International Lymphoma Radiation Oncology Group (ILROG). After individual institutional review board approvals (or equivalent in participating institutions), anonymized patient data were submitted to a single database according to a prospectively agreed protocol. Inclusion criteria were: grade 1 to 3A FL, stage I to II disease, staging that included 18F-FDG PET-CT, RT dose equivalent to at least 24 Gy in 12 fractions, posttreatment follow-up of ≥3 months, and no prior RT or prior or subsequent adjuvant systemic therapy. Pathology was confirmed at each individual institution before treatment.
We recorded age, sex, Eastern Cooperative Oncology Group performance status, race and ethnicity, disease stage and presence of B symptoms, nodal and extranodal sites of disease, maximum lesion size, histological grade, bone marrow biopsy, Follicular Lymphoma International Prognostic Index (FLIPI) score, and molecular markers when available [BCL2, BCL6, t(14;18)]. We also recorded details of RT, including dose, fractionation, field size, modality of treatment, and response to treatment.
Treatment, follow-up, and outcome assessment
RT was delivered via 2-dimensional, 3-dimensional conformal, intensity-modulated, and electron-beam modalities. Treatment volumes included involved field (IFRT), involved site (ISRT), and involved node RT (INRT).
The time interval until post-RT imaging (CT or PET-CT imaging obtained within 6 months) was recorded. Post-RT follow-up was defined from the end of RT. Post-RT surveillance approaches varied widely and included clinical follow-up only, CT alone, PET-CT, and endoscopy for duodenal sites. Post-RT PET-CT imaging was scored according to the Deauville 5-point scale.6
Treatment-related toxicities
Toxicities were graded retrospectively using clinic visit notations according to the National Cancer Institute Common Terminology Criteria for Adverse Events (version 4.03).
End points
The primary end point was freedom from progression (FFP), calculated from the date of RT completion to first progression based on clinical, radiographic, or pathologic evidence. Deaths were considered censor events. The secondary end points were local control, OS, and metabolic response rate on PET-CT.
Patterns of failure were recorded, with recurrence defined as distant (occurring outside) and local (within the RT target volume). Marginal recurrence was defined as within the same anatomical region but outside the RT target volume. For patients who relapsed, method of detection of relapse was recorded.
Statistical analyses
The Kaplan-Meier method was used to measure FFP and OS, with stratification evaluated using the log-rank test. Univariable and multivariable hazard ratios (HRs) were calculated using Cox regression analysis with corresponding Wald 95% confidence intervals (CIs) and P values. The proportional hazards assumption was tested by visualizing log(−log[survival probability]) plots. Patient factors meeting significance at P < .05 on univariable analysis were included in multivariable analysis, along with adjustment for other baseline characteristics.
Only 9 patients died without recurrent disease, with competing risk analysis demonstrating nearly identical results (supplemental Figure 1; supplemental Table 1, available on the Blood Web site). For the size of nodal disease, the largest recorded value (on examination or imaging) was used for statistical analysis, with imputation of missing values with the mean value. Statistical analysis was performed using SAS (version 9.3; SAS institute, Cary, NC) and R software (version 3.4; R Foundation, Vienna, Austria). All P values were 2 sided and considered significant at P < .05.
Results
Patient and treatment characteristics
A total of 512 patients treated from 2000 to 2017 at 16 centers were eligible for analysis. As shown in Table 1, a majority of patients had stage I disease (n = 410; 80.1%), underwent bone marrow biopsy (n = 479; 93.6%), and were without B symptoms (n = 507; 99%). Median follow-up was 52 months (range, 3.2-174.6 months; average, 59 months).
For patients with stage I disease, 297 (72.4%) had nodal disease only, including: cervical (which includes supraclavicular, occipital, and preauricular nodes; n = 102), axillary/pectoral (n = 26), mediastinal (n = 2), abdominal paraaortic (n = 5), mesenteric (n = 15), iliac (n = 4), inguinal/femoral (n = 140), and epitrochlear/brachial (n = 3). Extranodal sites in 113 patients (27.6%) included: Waldeyer’s ring (n = 13), parotid gland (n = 15), stomach (n = 2), bone (n = 7), skin (n = 10), breast (n = 6), thyroid (n = 3), orbit (n = 5), duodenum (n = 26), bladder (n = 1), soft tissue (n = 14), ileum (n = 1), colon (n = 1), other salivary gland (n = 3), and not otherwise specified (n = 6).
For patients with stage II disease, nodal sites were involved in all 102 patients and included: cervical (n = 40), axillary/pectoral (n = 20), mediastinal (n = 1), paraaortic (n = 9), mesenteric (n = 11), iliac (n = 26), and inguinal/femoral (n = 41). Only 8 patients with stage II disease had noncontiguous nodal sites. Extranodal disease was present in 14 patients (13.7%) with stage II disease and included: Waldeyer’s ring (n = 3), parotid gland (n = 2), bone (n = 2), duodenum (n = 2), breast (n = 2), thyroid (n = 1), lacrimal gland (n = 1), and larynx (n = 1).
Treatment was delivered via 2-dimensional (n = 5), 3-dimensional conformal (n = 315), intensity-modulated (n = 100), and electron-beam modalities (n = 15) and was unspecified in 77 patients. Median RT dose was 30 Gy (range, 24-52 Gy) delivered in a median of 2 Gy per fraction. The mode (most frequent dose) was 30 Gy, with 255 patients (49.8%) receiving 30 Gy; 167 patients received >30 Gy (32.6%), and as shown in Table 1, only a small percentage received >36 Gy (5.3%). Treatment volumes included IFRT (n = 256), ISRT (n = 144), and INRT (n = 7) and were unspecified in 105 patients.
Outcomes after definitive RT
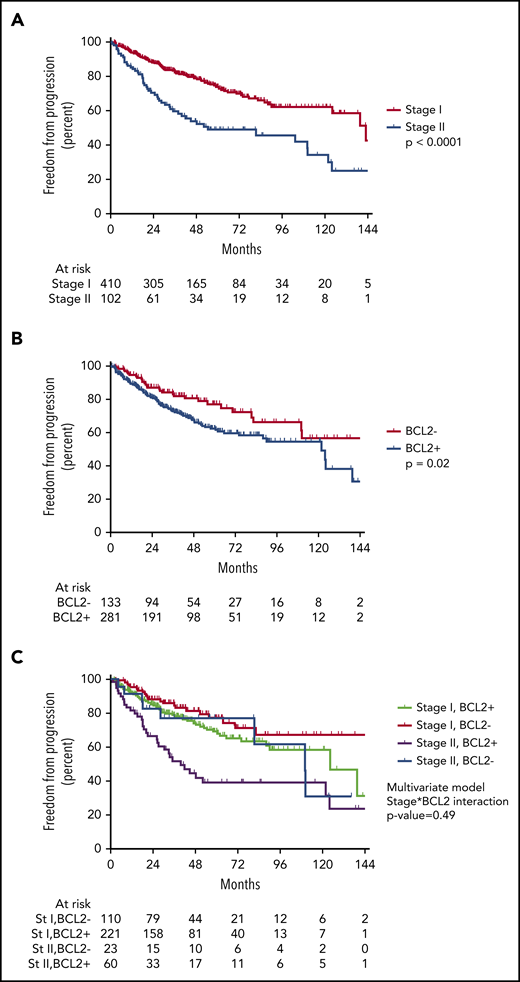
For the entire cohort, 5-year FFP was 68.9% (95% CI, 63.9%-73.4%; Figure 1A) and OS was 96.0% (95% CI, 93.2%-97.6%; Figure 1B). Only 8 patients relapsed in-field (1.6%), and 4 had marginal recurrences (0.8%), resulting in a local control rate of 97.6%; 137 (91.9%) relapses occurred outside of the irradiated sites. Five-year FFP for the 33 patients who did not undergo bone marrow biopsy was not significantly different from that for those who did but was numerically higher (81.3% vs 68.1%, respectively; P = .053).
Outcomes after primary RT for the entire cohort. (A) FFP with 5-year rate of 68.9%. (B) OS with 5-year rate of 96%.
Outcomes after primary RT for the entire cohort. (A) FFP with 5-year rate of 68.9%. (B) OS with 5-year rate of 96%.
There was no significant difference in FFP between patients treated according to ISRT or INRT criteria (total, n = 151; 29.4%) compared with patients treated with IFRT (P = .41). There was a significant difference in outcome by stage. Five-year FFP for stage I was 74.1% (95% CI, 68.5%-78.8%) vs 49.1% for stage II (95% CI, 37.8%-59.5%; P < .0001; Figure 2A).
FFP stratified by stage and BCL2 status. (A) FFP is significantly worse for patients with stage (st) II disease. (B) FFP is significantly worse for patients with BCL2+ expression. (C) Stratifying by stage and BCL2 status demonstrates patients with stage II BCL2− disease may have outcomes similar to those of patients with stage I disease.
FFP stratified by stage and BCL2 status. (A) FFP is significantly worse for patients with stage (st) II disease. (B) FFP is significantly worse for patients with BCL2+ expression. (C) Stratifying by stage and BCL2 status demonstrates patients with stage II BCL2− disease may have outcomes similar to those of patients with stage I disease.
There was no significant difference in 5-year FFP between nodal and extranodal presentations (P = .34; supplemental Figure 2), including when stratified by stage (log-rank P = .63 for stage I and P = .92 for stage II). There were no recurrences for the 28 patients with duodenal involvement.
On univariable analysis, stage was significantly associated with higher risk of progression (HR, 2.34; 95% CI, 1.66-3.30; P < .0001; Table 2). On multivariable analysis, stage II remained significantly associated with higher risk for progression (HR, 2.26; 95% CI, 1.60-3.19; P < .0001; Table 2) after adjusting for baseline patient characteristics, including sex, stage, extranodal status, and FLIPI score.
A majority of patients had molecular marker testing available (n = 414; 80.9%). Of those without BCL2 status, 73.5% (72 of 98) were from a single center that did not collect molecular data as part of its prospective database collection. Five-year FFP was significantly worse for patients with BCL2+ expression compared with those without expression (BCL2−; 62.5%; 95% CI, 55.3%-68.9% vs 77.2%; 95% CI, 67.3%-84.5%, respectively; P = .02; Figure 2B).
On univariable analysis, BCL2+ was significantly associated with higher risk of progression (HR, 1.64; 95% CI, 1.09-2.46; P = .02; Table 2). On multivariable analysis, both BCL2+ and stage II remained independently associated with higher risk for progression (Table 2) after adjusting for baseline patient characteristics, including sex, stage, extranodal status, and FLIPI score. There was no significant interaction between stage and BCL2 expression when tested in the multivariable Cox model (interaction P = .49; Figure 2C).
Imaging response to RT
Within 6 months after completion of RT, 273 patients (53.3%) were assessed with either PET-CT or CT; 107 (20.8%) underwent a CT scan, performed at a median of 2.8 months (range, 0.6-5.8 months; IQR, 1.9-3.5 months); 166 (32.4%) had a PET-CT scan to assess response; this was at a median of 2.9 months from treatment (range, 0.6-6 months; IQR, 1.8-3.5 months).
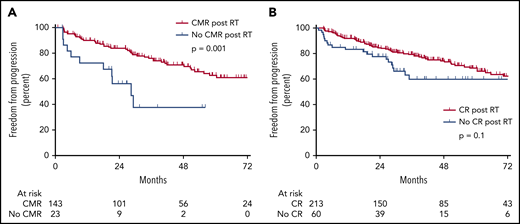
On PET-CT, 143 (86.1%) achieved complete metabolic response (CMR; Deauville score, 1-3). Failure to achieve CMR (n = 23; 13.9%) was associated with higher risk of progression (P = .001; Figure 3A). Twenty-three patients did not achieve a CMR, of whom 10 (43.4%) ultimately developed recurrent disease, all occurring distantly to the radiation field. Thirteen patients (56.5%) did not develop recurrent disease; in this group, 4 had subsequent normalization of FDG uptake, 7 had stable disease or CR on subsequent CT, and 2 had no recurrence based on clinical follow-up. Failure to achieve CR by size criteria on CT was not significantly associated with a higher rate of progression (P = .1; Figure 3B).
Ability of post-RT imaging to predict recurrence. (A) Patients without CMR after primary RT have a significantly higher rate of progression. (B) Patients without CR after primary RT have a nonsignificantly higher rate of progression.
Ability of post-RT imaging to predict recurrence. (A) Patients without CMR after primary RT have a significantly higher rate of progression. (B) Patients without CR after primary RT have a nonsignificantly higher rate of progression.
We found no significant difference when comparing time to relapse for those with and without post-RT PET, with median time to relapse of 21.1 months (range, 0.9-123.9 months) vs 24.6 months (range, 1.6-142 months), respectively (log-rank P = .25). When comparing the patient characteristics between those with and without post-RT PET, we did not find any significant difference between age, sex, stage, extranodal status, or size (supplemental Table 2).
Detection of relapse
The first method of detection of recurrent disease (n = 149) included: surveillance imaging (n = 55; 36.9% of all relapses), patient symptoms (n = 34; 22.8% of all relapses), clinical examination (n = 11; 7.4% of all relapses), or other/unknown (n = 49; 32.9% of all relapses). We found a nonsignificantly higher rate of relapse detected by imaging for patients with initial abdominal or pelvic (noninguinal) involvement vs all other sites of involvement (21.8% [12 of 55] vs 12.7% [12 of 94]; P = .15).
Toxicity
Acute and late toxicity data were available for 372 patients (72.7%). Eighty-five patients (22.8%) experienced the following grade 1 to 2 acute toxicities: diarrhea (n = 6), abdominal pain (n = 1), esophagitis (n = 1), radiation dermatitis (n = 25), fatigue (n = 11), xerostomia (n = 8), nausea (n = 8), limb edema (n = 2), increased urinary frequency (n = 2), dry eye (n = 1), taste alteration (n = 4), mucositis (n = 18), dysphagia (n = 9), weight loss (n = 1), alopecia (n = 2), and not otherwise specified (n = 9). Grade 3 toxicities were rare and included dysphagia (n = 1), dehydration (n = 1), and mucositis (n = 1). Late toxicities included grade 1 dry mouth (n = 1) and grade 2 hypothyroidism (n = 1).
Second malignancies occurred in 2.1% (n = 11) of patients and included cutaneous melanoma, de novo metastatic melanoma (n = 2), ductal carcinoma in situ of the breast, endometrial cancer (n = 2), colorectal adenocarcinoma (n = 2), neuroendocrine carcinoma, acute myeloid leukemia, and clear cell renal carcinoma. All except the ductal carcinoma in situ of the breast occurred outside of the prescription RT field.
Discussion
This multiinstitutional study is the largest to evaluate outcomes after RT for patients with stage I or localized stage II FL who underwent modern staging including 18F-FDG PET-CT. As PET-CT staging results in more accurate staging and a significant incidence of upstaging from limited stage (I/II) to advanced stage (III/IV), we hypothesized that the improved selection criteria would result in improved outcomes for patients with stage I/II disease.
In this study, we included only patients who had a staging PET-CT and were treated with definitive RT with conventionally fractionated doses of ≥24 Gy. We did not include other patients with stage I or localized stage II who received other or no treatment. Median follow-up was 52.3 months. With an estimated 5-year FFP of 68.9% (74.9% for stage I and 49.1% for localized stage II) and OS of 96%, treatment results in this study at this time point are considerably better than those reported in historical series from the pre–PET-CT era.
One of the earliest series was from Stanford University and included 177 patients treated between 1961 and 1994.3 This reported 5- and 10-year freedom from relapse rates of 55% and 44%, with 5- and 10-year OS rates of 82% and 60%, respectively. Staging for these patients included bipedal lymphangiography and bone marrow trephine biopsy, with CT scanning only in the later cases; 25% of patients underwent staging laparotomy.
The Stanford results were in line with those of a larger study from Princess Margaret Cancer Centre in Toronto, Canada. The study evaluated 460 patients treated between 1968 and 1999. Relapse-free rates at 5 and 10 years posttreatment were 62% and 52%, respectively, with 5- and 10-year OS rates of 79% and 62%.14 Similar outcomes were observed in several other single-institution studies published in the 1990s and early 2000s.15-17
As anticipated, local control post-RT in our study was excellent. Fewer than 2% of patients experienced in-field relapses. A great majority of relapses, 91.9%, were at sites beyond the irradiation field. This high degree of local control and relapse pattern are consistent with other reports. In the 24-Gy arm of the FORT study, only 21 (7%) of 299 patients progressed in field, with a median follow-up of 26 months.18 In the PMH series, the in-field relapse rate was 5.5%,14 which is almost identical to the Stanford result of 5%.19 In a retrospective study of 80 patients with stage I to II FL treated from 1960 to 1988 at the MD Anderson Cancer Center, the 15-year local control rate was 100% for tumors <3 cm and 93% for those ≥3 cm.4
For patients with stage I disease in our series, outcomes after RT were particularly good, with estimated 5-year FFP of 74.9%. In stage II disease, the relapse rate was higher; however, half of the patients remained disease free at 5 years, with estimated 5-year FFP of 49.1% (P < .0001). OS at 5 years in both groups was 96%.
The proportion of patients with stage II disease in our study was much smaller than in older series. This could be due to the upstaging effect of PET-CT, in that patients previously deemed to have stage II disease were found to have stage III/IV disease on PET. In addition, because all these patients were treated after 2000, it is possible that fewer patients with stage II disease were offered RT in favor of systemic treatment or observation, a trend that has been reported in more recent years.20
Site of disease (nodal vs extranodal) did not correlate with risk of relapse, but of note, our cohort included 28 patients with FL involving the duodenum. Duodenal FL is increasingly recognized to have a favorable prognosis, and similar to experiences reported by others, we observed excellent outcomes for these patients, without any recurrences during follow-up.21
In a multivariable model, the only other factor associated with risk of relapse in addition to stage was BCL2 expression. Patients with BCL2-expressing tumors were significantly more likely to relapse. Interestingly, the outcomes of patients with stage II FL who were BCL2− were similar to those of patients with stage I disease. However, there were only 23 patients in this group, and this may not have provided sufficient power to detect any possible interaction between stage and BCL2.
BCL2 overexpression (BCL2+) is present in ∼80% to 90% of cases of FL,16,22,23 and although it confers a poor prognosis in diffuse large B-cell lymphoma, a relationship between BCL2+ and outcome in FL has not been established.24,25
The incidence of BCL2+ in our study was lower than other series with cases of more advanced disease. Of the 414 patients with known BCL2 status, only 281 (68%) were BCL2+. This is likely related to the early stage of disease. One other study evaluated patients with stage I or II FL treated with RT, combined-modality therapy, or chemotherapy alone and reported BCL2+ in 73% of patients, which is more keeping with our results. However, that study did not identify any relationship between BCL2 status and outcome.26 The t(14;18) translocation, the hallmark of FL, has also been shown to occur less frequently in those with early- compared with advanced-stage disease. In a study of 174 patients with grade 1 to 3A FL, those who were t(14;18) − were significantly more likely to have localized disease compared with patients with translocation (62% vs 32%).23 Although we cannot make any firm conclusion regarding BCL2+ and prognosis of early-stage disease treated with RT based on this study, we consider the findings to be hypothesis generating and worth of further investigation.
The high local control after RT and the fact that most relapses were distant suggest the presence of microscopic disease below the threshold of PET detection in a quarter of patients with stage I and half of patients with stage II disease. This raises the question of whether the addition of systemic therapy would have improved outcomes further. In a retrospective series from the University of Torino, the addition of single-agent rituximab improved results compared with historical controls.27 A more recent randomized controlled study testing the addition or chemotherapy or chemoimmunotherapy showed statistically significantly improved progression-free survival, although longer follow-up is required to evaluate any benefit in OS.28 Therefore, combined-modality therapy may be a useful strategy, particularly for those with stage II disease, who are at significantly higher risk of relapse after local treatment.
According to international guidelines, PET-CT in lymphoma is used for both initial staging and response assessment.5,6 In our study, 32.4% of patients underwent response assessment with PET-CT. This was defined as a PET-CT within 6 months of completion of treatment. For this subgroup, achieving a CMR, defined as Deauville score of 1 to 3, was strongly associated with a decreased risk of relapse. Patients with incomplete metabolic response were nearly 43 times more likely to relapse than those achieving CMR. Of the patients who did not achieve a CMR, approximately half developed recurrent disease, all failing distantly outside of the irradiation field. The other half remained in remission, with subsequent normalization of PET or no evidence of progression on CT or clinically. These results suggest that PET-CT response after RT is worth exploring further to elucidate whether a high-risk subset of patients who might benefit from additional systemic treatment can be identified. Posttreatment CT scanning was not found to predict outcome.
Early toxicity data were available for most patients (72.7%), and overall toxicity from RT was minimal, with only 3 cases of grade 3 toxicity (dysphagia, dehydration, and mucositis). There were no treatment-related deaths. These results are not dissimilar to the prospective data from the 24-Gy arm of the FORT trial, in which only 2.8% of patients experienced grade 3 and no patients grade 4 toxic effects from treatment.18 Late toxicity data collection was limited by the short follow-up of the study and its retrospective nature, which is a limitation of this study.
The excellent tolerability of RT is an important consideration in making treatment decisions, particularly because some have suggested that a watch-and-wait policy is appropriate for these patients with potentially curable limited-stage FL.29 Our results show that most patients do not experience significant adverse effects after the relatively low doses and limited irradiation fields employe,d and they benefit from the chance of cure offered only by RT.
The main limitation of our study is its relatively short follow-up for a disease with a long natural history. Data from historical series suggest that additional relapses can occur beyond 5 years, with an incidence in the order of 10% from 5 to 10 years.3,14,19 Longer follow-up is required to confirm the long-term outcomes.
Other limitations are that there was no central pathology or PET-CT review, although all patients in our study were treated in specialist academic centers with significant expertise in treating hematological malignancies. Given the long inclusion period during which patients were treated, there was variation in RT dose, technique used, and volume treated (ie, involved field, site, and node). We did not identify any relationship between these treatment factors with regard to RT or risk of progression. However, we would consider the 2014 ILROG guidelines for lymphoma RT to be the current international standard.30
A small group (6.4%) of patients in our study did not have a bone marrow biopsy pre-RT as per international guidelines. These patients had similar FFP to those who underwent biopsy. In other studies, however, rigorous staging has consistently been shown to be associated with better treatment outcomes.31-33 We still believe bone marrow biopsy to be standard of care.
The improved outcomes seen in this study support RT as an excellent treatment option in localized FL, particularly for patients with stage I disease. Several studies, however, have suggested that upfront RT is being underutilized in patients with limited-stage FL, which contradicts international guidelines. In the LymphoCare study, only 23.4% of patients with stage I disease received RT as their initial treatment.31 Analysis of data from the Surveillance, Epidemiology, and End-Results (SEER) database for 6568 patients with stage I/II FL diagnosed between 1973 and 2004 showed that only 34% received RT.33 A more recent study of 35 631 patients in the National Cancer Database with grade 1 to 2 localized FL treated between 1998 and 2012 showed that RT use had decreased from 37% in 2009 to 24% in 2012.20
Critics of RT may argue that survival in all groups is high and that there are no randomized data comparing upfront RT with primary chemoimmunotherapy or surveillance; however, the SEER database analysis (6568 patients) showed that initial treatment with RT was independently associated with both improved disease-free survival and OS, with an absolute benefit of 13% in OS for RT-treated patients.33 This was also found in the National Cancer Database study (35 631 patients), with upfront RT remaining independently associated with improved OS (HR of death, 0.54; 95% CI, 0.47-0.63; P < .0001). The authors of both studies concluded that RT was underused.
Bearing in mind the low toxicity of modern RT, it should be considered as an initial treatment option for limited-stage FL in suitable patients. Our study suggests that it is a highly effective treatment, with nearly three-quarters of patients with stage I and approximately half of patients with selected localized stage II disease remaining disease free at 5 years. Although longer follow-up is clearly needed, it is likely that earlier series in the pre–PET-CT era underestimated the value of RT as a curative treatment of stage I/II FL due to the limited sensitivity of the staging techniques. Long-term outcomes after the addition of systemic therapy to RT are eagerly awaited to see if this improves outcomes further.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: J.L.B. as first author designed the study, collected data, analyzed data, and wrote the first draft of the manuscript; M.S.B. as joint first author designed the study, analyzed data, performed statistical analysis, and wrote the first draft of the manuscript; R.T.H. and N.G.M. designed the study, collected data, analyzed data, and wrote the manuscript; and C.H., M.C., K.C., A.B., M.L., A.R.F., M.J., M.M.M., A.W., M.O., A.K.V., T.Y.A., A.K.N., B.M.P.A., S.H.C., Y.K., S.H., G.R., H.T.E., S.V.B., L.S.C., C.-O.S., B.D., T.C.E.-G., D.C.H., U.R., and J.Y. collected data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A list of the steering committee members of the International Lymphoma Radiation Oncology Group appears in “Appendix.”
Correspondence: Jessica L. Brady, Guy’s Cancer Centre, Guy’s and St Thomas’s Hospital, Great Maze Pond, London, SE1 9RT United Kingdom; e-mail: jessica.brady@gstt.nhs.uk.
Appendix: study group members
The steering committee members of the International Lymphoma Radiation Oncology Group are: J.Y., Lena Specht, B.M.P.A., Anne Kiil Berthesen, L.S.C., B.D., Karin Dieckmann, H.T.E., N.G.M., D.C.H., Stephanie Terezakis, R.T.H., Tim Illidge, Y.K., Ye-Xiong Li, C.-O.S., Siddhartha Laska, A.K.N., M.O., John P. Plastaras, U.R., Richard W Tsang, Lynn D. Wilson, and Andrew Wirth.
REFERENCES
Author notes
J.L.B. and M.S.B. contributed equally to this work.