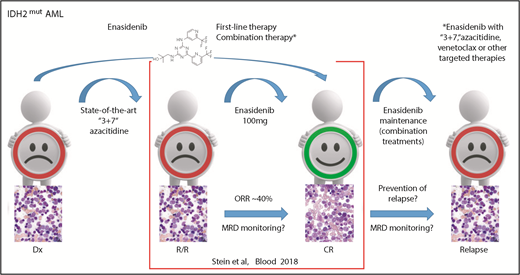
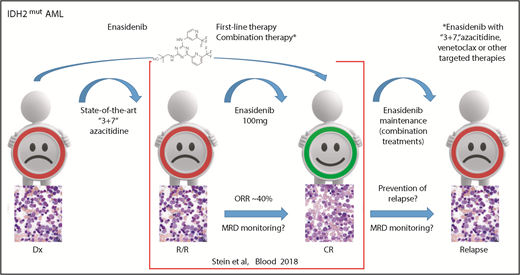
In this issue of Blood, Stein et al1 report the outcomes for all isocitrate dehydrogenase-2 (IDH2) mutant relapsed or refractory acute myeloid leukemia (R/R AML) patients treated with enasidenib in the first phase 1/2 study including data on molecular clearance and molecular relationships to response or resistance.
IDH2 mutant R/R AML responds to enasidenib monotherapy inducing a CR in ∼20% of patients. Measurable residual disease (MRD) monitoring will be helpful in predicting treatment response because response may occur as late as after the sixth cycle. Using enasidenib in first-line therapy or in combination with other therapies might further improve outcome and avoid resistance. These strategies will be further evaluated in ongoing studies, including maintenance therapy. Dx, diagnosis; “3+7,” standard chemotherapy with anthracycline and cytarabine. Bone marrow morphology images adapted from Stein et al.5
IDH2 mutant R/R AML responds to enasidenib monotherapy inducing a CR in ∼20% of patients. Measurable residual disease (MRD) monitoring will be helpful in predicting treatment response because response may occur as late as after the sixth cycle. Using enasidenib in first-line therapy or in combination with other therapies might further improve outcome and avoid resistance. These strategies will be further evaluated in ongoing studies, including maintenance therapy. Dx, diagnosis; “3+7,” standard chemotherapy with anthracycline and cytarabine. Bone marrow morphology images adapted from Stein et al.5
Following a long dry spell, novel targeted therapeutic options are changing AML management, most notably midostaurin added to standard chemotherapy with anthracycline and cytarabine for FLT3 mutant AML.2 Technological advances are improving the molecular characterization of AML. Studies in large well-characterized patient cohorts could provide further evidence that AML with IDH2 R172 mutation might represent a distinct disease class, based on the mutual exclusivity of NPM1 mutation and other class-defining lesions.3
Mutated IDH2 is found at position R172 in ∼1% to 4% of AML patients. Mutations at R140 are found in ∼5% to 15% of AML cases, which are often accompanied by NPM1 mutations.4 Both IDH2 mutation positions represent early events in leukemogenesis and both positions are associated with clonal hematopoiesis in healthy elderly people. IDH2 mutation incidence increases with age (∼5% to 10% in patients <60 years; ∼10% to 15% in patients >60 years).2
Recently, inhibitors have been developed for mutant cytoplasmic IDH1 and mitochondrial IDH2, providing important new pieces for the leukemia treatment puzzle. The first results for enasidenib, AG-221, a first-in-class orally available inhibitor of mutant IDH2, in R/R AML showed an overall response rate (ORR) of 40% last year.5 Results from the first clinical study of the inhibitor of mutant IDH1, ivosidenib, AG-120, reported a similar ORR of 41.6% earlier this year.6
In this issue of Blood, Stein and colleagues provide an update of the enasidenib study of 214 R/R AML patients (median age of 68) treated at a dose of 100 mg/d. This report confirmed the high rate of complete remission (CR) in 19.6% of patients with 10.3% proceeding to an allogeneic bone marrow transplant. Thus, enasidenib monotherapy demonstrated high efficacy with an ORR of 38.8% (95% confidence interval [CI] 32.2-45.7) and a median overall survival (OS) of 8.8 months (95% CI 7.7-9.6). Notably, similar to epigenetic treatment, the best response was seen quite late (ie, by cycle 4 in 46% and by cycle 6 in 80% of responding patients). This suggests that molecular monitoring will be important in this AML subgroup, as molecular response might be detected before clinical response and can be used to better guide targeted IDH2 treatment (see figure). Reduction in IDH2 variant allele frequency and molecular clearance with enasidenib were associated with attainment of CR. Similarly, the magnitude of 2-hydroxyglutarate reduction on-study was associated with CR in IDH2-R172 patients.
Although response rates were similar among patients with relapsed (37.7%) and refractory disease (37.5%), recent data presented at the 2018 Annual Meeting of the American Society of Hematology Meeting in San Diego point to an even higher effectiveness in first-line AML therapy.7 In the current study of R/R AML, clinical activity of enasidenib was negatively affected by baseline comutations in patients with FLT3-ITD or -TKD as well as NRAS mutations. Similarly, in previously untreated cases, RAS or PTPN11 were also found unfavorable.7
Enasidenib monotherapy is well tolerated, and the most common grade 3 to 4 treatment-related adverse events were hyperbilirubinemia in 10%, thrombocytopenia in 7%, and IDH differentiation syndrome in 6% of cases. This suggests that enasidenib might also be well tolerated in combination therapies. Initial data provide evidence for compatibility with azacitidine,7 and with conventional chemotherapy in first-line therapy.8
Given that not all IDH2 mutant patients respond to IDH2 inhibition, combination treatment with state-of-the-art conventional chemotherapy and azacitidine, as well as combined with novel AML treatment strategies, such as tyrosine kinase and BCL2 inhibitors, will be important to further improve outcome (see figure). This is of special importance because recently mechanisms of acquired resistance to targeted IDH2 therapy have been demonstrated by the detection of second-site IDH2 mutations.9 However, relapse can also arise by clonal evolution or selection of ancestral clones.10 Thus, combined targeting of several potential bypass pathways in parallel could be used to increase CR and OS rates.
In summary, Stein and colleagues provide further evidence that IDH inhibition may be an important addition in the leukemia treatment armamentarium with the power to induce remissions and hematologic responses in patients with AML for whom prior treatments had failed, especially in older AML patients. In the future, individualized combination treatment strategies will include enasidenib for IDH2 mutant patients, thereby adding more pieces to better solve the molecular puzzle of AML.
Conflict-of-interest disclosure: L.B. declares no competing financial interests.