Abstract
T-cell prolymphocytic leukemia (T-PLL) is a rare, mature T-cell neoplasm with a heterogeneous clinical course. With the advent of novel treatment options that will potentially change the management of patients with T-PLL, it has become necessary to produce consensus guidelines for the design and conduct of clinical trials. The T-PLL International Study group (TPLL-ISG) set out to define standardized criteria for diagnosis, treatment indication, and evaluation of response. These criteria will facilitate comparison of results from clinical trials in T-PLL, and will thus support clinical decision making, as well as the approval of new therapeutics by healthcare authorities.
Introduction
In 1973, T-PLL had been recognized as distinct from chronic lymphocytic leukemia (CLL) by its specific clinical presentation, the morphological characteristic of affected lymphoid cells, and the poor clinical outcome.1 Modern oncology has witnessed an increasing number of new effective anticancer drugs; however, so far, they have limited benefit for patients with T-PLL. With an overall incidence of only 0.6/Mio in the general population, T-PLL is a very rare disease.2,3 Because of the rapidly progressing clinical course and often uncertain diagnosis, it has been a difficult disease to approach systematically. Case reports, single-center retrospective studies, and small trials are difficult to compare because of poorly defined and inconsistent diagnostic criteria and response evaluation.4,,,,-9 So far, T-PLL has been managed according to criteria for CLL, as described by the international workshop on CLL (IWCLL).10,,-13 T-PLL is biologically and clinically very different from CLL, and the IWCLL criteria are not always appropriate. Furthermore, modifications of the IWCLL criteria adopted by investigators have not been uniform. Thus, there is an unmet need for disease-specific criteria for the establishment of diagnosis and response evaluation to provide a basis to compare results and to systematically advance treatment strategies for T-PLL. To define T-PLL-specific consensus standards for the design and conduct of clinical trials, the T-PLL International Study Group (TPLL-ISG) assembled in May 2017 in Vienna, Austria. In the following months, the group agreed on a blueprint consensus paper that aims to provide definitions to be used in routine clinical practice and upcoming studies, eventually paving the way for harmonization of data that will come from future clinical trials.
Diagnosis of T-PLL
The 2017 World Health Organization classification defines T-PLL as aggressive T-cell leukemia of proliferating small to medium-sized T-lymphocytic cells that, despite their postthymic origin and mature phenotype, are called T-prolymphocytes.1,14 T-PLL includes the formerly used category of T-cell CLL.
T-PLL represents 2% of chronic leukemias in adults.2,3 The largest observational studies that described clinicopathologic features at presentation included between 38 and 119 T-PLL cases, indicating series with more than 50 T-PLL cases should be considered to be very large.2,3,15,16 Clinical presentation includes B-symptoms, hepato-splenomegaly, and usually excessive lymphocytosis above 100 × 109/L. Nodal and extranodal presentation is also frequent, including skin, pleural or peritoneal effusions in around 25% of patients, and central nervous system involvement in less than 10% of patients.2,14 Periorbital, conjunctival edema and peripheral edema are frequently observed in patients with T-PLL.2
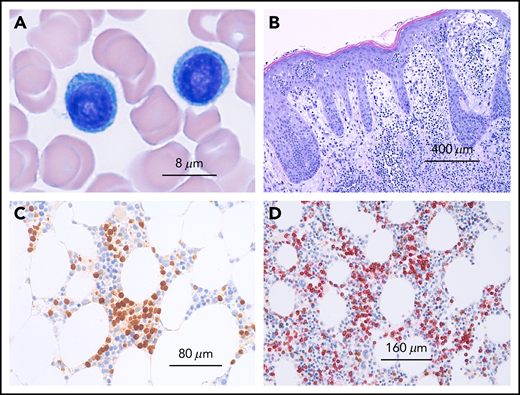
T-PLL is a molecularly and clinically distinct disease that has to be discriminated from other leukemic T-cell neoplasia demonstrating overlapping features, such as Sézary syndrome, T-cell large granular lymphocytic leukemia, adult T-cell lymphocytic leukemia, or other (nodal) mature T-cell tumors with marked leukemic presentation.3,16,17 To verify the correct diagnosis, it is necessary to evaluate peripheral blood smear (morphology; Figure 1), immunophenotype, and genetic features (Figure 2), although in most of the cases, the diagnosis can be established using only morphology and immunophenotype of peripheral blood lymphocytes. Bone marrow evaluation is required for treatment evaluation (confirmation of a remission), but is not usually required to confirm the diagnosis. Table 1 lists recommended assessments at diagnosis and during the course of the disease. The diagnosis of T-PLL is established if all 3 major criteria are met or if the first 2 major criteria and 1 minor criterion are met (TCL1-family negative T-PLL; Table 2). As discussed in more detail here, the first 2 major criteria are T-PLL-defining characteristics.2,3,17 The third major criterion, alterations of TCL1A, TCL1B (TML1), or MTCP, is present in more than 90% of cases; however, it is not present in 100%, and therefore at least 1 of the less common genetic features or T-PLL typical site involvements has to be present.3
Microscopy of T-cell prolymphocytic leukemia. (A) Peripheral blood smear showing 2 medium-sized atypical lymphocytes with round nuclei and prominent nucleoli and abundant basophilic cytoplasms without granules. (B) Cutaneous involvement in T-PLL. Dense dermal infiltrates with marked epidermotropism simulating mycosis fungoides. (C) Bone marrow infiltration by T-PLL with an interstitial infiltration pattern depicted by an immunohistologic stain with an antibody against TCL1. (D) T-cell origin of TCL-1+ cells confirmed by immunohistology by double staining with CD5 (red) and TCL1 (brown).
Microscopy of T-cell prolymphocytic leukemia. (A) Peripheral blood smear showing 2 medium-sized atypical lymphocytes with round nuclei and prominent nucleoli and abundant basophilic cytoplasms without granules. (B) Cutaneous involvement in T-PLL. Dense dermal infiltrates with marked epidermotropism simulating mycosis fungoides. (C) Bone marrow infiltration by T-PLL with an interstitial infiltration pattern depicted by an immunohistologic stain with an antibody against TCL1. (D) T-cell origin of TCL-1+ cells confirmed by immunohistology by double staining with CD5 (red) and TCL1 (brown).
Immunophenotypic markers of T-PLL and typical chromosome banding analysis. (A) Flow cytometry of CD3+, CD5+, cytoplasmic TCL1+ T-PLL cells. (B) Two representative T-PLL chromosome banding analyses.
Immunophenotypic markers of T-PLL and typical chromosome banding analysis. (A) Flow cytometry of CD3+, CD5+, cytoplasmic TCL1+ T-PLL cells. (B) Two representative T-PLL chromosome banding analyses.
Blood and bone marrow
The diagnosis of T-PLL requires the presence of clonal prolymphocytic T cells in the peripheral blood or bone marrow, which can present in 3 morphologic variants1,2,14,18,-20 : most commonly (75%), T-PLL cells are medium-sized with a high nuclear/cytoplasmic ratio, moderately condensed chromatin, a single visible nucleolus, and a slightly basophilic cytoplasm without granules but typically demonstrating cytoplasmic protrusions (blebs); in 20%, T-PLL present as a small cell variant with condensed chromatin and a nucleolus that is invisible by light microscopy; and in about 5% of cases, T-PLL demonstrates irregular nuclei similar to the cerebriform nuclei found in Sézary cells of mycosis fungoides (cerebriform variant). These variants are not associated with distinct clinical presentations or with outcomes.
The clonality of these T lymphocytes should be confirmed by polymerase chain reaction (PCR; or next-generation sequencing [NGS]-based assessment) of the clonal rearrangement of T-cell receptor genes (TRB and TRG) or by flow cytometry.20 However, it is to the physician’s discretion to judge whether an explicit test for clonality might be redundant in certain situations. Serologic or PCR-based testing for HTLV (human T lymphotropic virus) type 1 is negative (to distinguish from adult T-cell lymphocytic leukemia).2,14,17,-19
Infrequently, asymptomatic patients are diagnosed as part of the evaluation of an abnormal laboratory test result. A bone marrow examination is not generally required to establish the diagnosis of T-PLL; however, it may be important to elucidate unclear cytopenias. Evaluation of the bone marrow (aspirate and biopsy) is mandatory to confirm a complete remission (CR) and complete remission with incomplete recovery (CRi).
Immunophenotype
Genetics
T-cell receptor genes (TRB or TRG) are clonally rearranged in T-PLL cells, which should be confirmed (by PCR/ NGS or flow cytometry20 ). Patients with T-PLL commonly demonstrate complex karyotypes (in 70%-80%) with recurrent genetic features that help establish the diagnosis.2,21,-23 Therefore, chromosome banding analysis and fluorescence in situ hybridization (FISH) can help to distinguish T-PLL from other T-lymphoproliferative disorders. Rearrangements involving TCL1 (T-cell leukemia/lymphoma1) family genes TCL1A, MTCP1 (mature T-cell proliferation), or TCL1B (alias TCL1/MTCP1-like 1 [TML1]), are relatively specific for T-PLL and are present in more than 90% of cases, either as inv(14)(q11q32) or t(14;14)(q11;q32) (involving TCL1A or TCL1B), or t(X;14)(q28;q11) (involving MTCP1; mature T-cell proliferation). Detection of aberrant TCL1 protein expression via flow cytometry or immunohistochemistry is more sensitive than cytogenetics and also represents a diagnostic hallmark.3,22,24,25 Few cases are observed in which neither a rearrangement nor an overexpression of TCL1A, TCL1B, or MTCP1 is detected, but which otherwise carry typical clinical, cytomorphological, and molecular criteria of T-PLL (clonal expansion of cells with a prolymphocytic T-cell phenotype). These cases should be collected for a more comprehensive scientific analysis and labeled as TCL1-family negative T-PLL.
Deletions of or missense mutations at the ataxia telangiectasia mutated (ATM) locus at 11q23 are found in up to 80% to 90% of T-PLL cases.16,26,-28 Patients with ataxia telangiectasia who harbor ATM germ line mutations have an increased risk of developing T-PLL.27,28 Other chromosomal or genetic abnormalities of sporadic T-PLL include chromosome 8 (idic(8)p11), t(8;8)(p11-12;q12), and trisomy 8q),29 deletion in 12p,30 and abnormalities in chromosome6 in 33% of cases.31 Deletions of the TP53 gene are observed in 31% of cases, and TP53 mutations in 14% of cases in T-PLL.32
NGS has identified additional recurrent genomic abnormalities, many involving JAK/STAT signaling (up to 75% of cases).33,-35 JAK3 mutations demonstrated a significant negative effect, and are so far the only genetic alteration of putative prognostic significance in T-PLL.32
Other common genetic lesions are haploinsufficiency for the CDKN1B tumor suppressor gene (half of cases30,36 ) and mutations in the epigenetic regulators EZH2 (deletions, 18%; mutations, 13%), TET2 (17%), and BCOR (9%), none of which are specific for T-PLL.35 Taken together, assessment of clonal TCR rearrangement, cytogenetics, and FISH are relevant genetic tests to establish the diagnosis T-PLL. Genetic sequencing is currently not a diagnostic requirement; however, it may provide information regarding the underlying pathogenesis of T-PLL or might help to identify relevant prognostic subgroups.
Serum markers
Similar to other lymphoproliferative disorders, serum lactate dehydrogenase (LDH) and β 2 microglobulin (B2MG) may reflect disease burden in T-PLL. In a retrospective study of 119 patients with T-PLL, LDH higher than 1668 U/L and B2MG higher than 8 mg/L were associated with inferior overall survival (OS).15 High LDH levels were also associated with a shorter OS in multivariate analysis.15 Elevated B2MG together with lymphadenopathy and organomegaly were suggestive of bulky disease.15 Both LDH and B2MG might therefore indicate tumor mass, and should be used in prospective clinical trials to validate their relative value in disease staging and further patient management.
Clinical staging and indication for treatment
Most patients with T-PLL present with an elevated white blood cell count (typically >100 × 109/L), splenomegaly, hepatomegaly, and generalized lymphadenopathy.2,17 However, up to 20% to 30% of patients with T-PLL demonstrate initially stable or slowly progressive disease.37 These 2 different disease states are relevant for the clinical management of patients. Criteria for initiating treatment in a clinical trial and in general practice are the same as those that define active disease. Because there is no evidence that asymptomatic patients with T-PLL with inactive disease (“inactive T-PLL”) would benefit from immediate treatment, it should be restricted to patients with active or symptomatic disease (“active T-PLL”). For inactive T-PLL (indicating an early phase and avoiding the misleading term “indolent”), a period of observation is recommended unless there is disease progression or development of disease-related symptoms (Table 4). Note, however, that within 1 to 2 years, nearly all of inactive T-PLL convert into active disease. During the observation period, blood counts should be performed at monthly intervals along with a clinical examination. A blood lymphocyte doubling time less than 8.5 months has been associated with a worse prognosis.22 Provided the initial T-PLL cell count is greater than 30 000/μL, lymphocyte doubling time of less than 6 months or an increase of more than 50% within 2 months indicate active T-PLL (in analogy to CLL as 1 criterion among others).12
Table 1 lists the recommended assessments before a treatment is commenced. An evaluation of comorbidities as established for patients with CLL by using the cumulative illness rating scale is recommended before the start of treatment, as it might be relevant for treatment choice, dosing, and assessments of response to therapy.38 Treatment is indicated for patients with active T-PLL and any of the listed disease-related symptoms (Table 4). This recommendation is generally for primary treatment decisions, as well as for second- and subsequent-line decisions; however, recurrent disease rarely presents as inactive T-PLL.2,3,5,17
Definition of response, relapse, and refractory disease
Response evaluation needs to monitor the actual state of disease, as well as the performance status. Assessments include a careful physical examination, blood tests, radiology, and confirmation of remission by evaluation of bone marrow (both aspirate and trephine; Table 1). Because response kinetics and recovery of normal cells depend on characteristics and modalities of specific treatment strategies, a general recommendation on adequate timing of response, assessments cannot be made. Therefore, timing needs to be evaluated individually for chemotherapeutics, which are administered in a defined treatment period; for antibodies; and for small molecule inhibitors, the latter of which are often more continuously administered. For continuous therapeutic strategies, response assessment should be performed at predefined times and treatment interruptions are not considered to be required for response assessment. In analogy to CLL, 2 groups of parameters should be assessed to define a response to a given therapy.12 Group A parameters evaluate constitutional symptoms and direct parameters of T-PLL (tumor load), group B parameters evaluate indirect effects of T-PLL cells on the function of the hematopoietic system (Table 5).
No specific response criteria for imaging tests such as computed tomography (CT) are currently established for T-PLL. Although adoption of the iwCLL12 or Lugano39 criteria that are used for response assessment in patients with CLL and patients with lymphoma might be an option, both systems rely on bidimensional measurements (ie, the sum of the products of long- and short-axis diameters) of up to 6 target lesions, which is a time-intensive approach that may be impractical for clinical use. Recently, the International Working Group proposed RECIL, a variation of RECIST 1.1, as a simple alternative to the Lugano criteria for response assessment in lymphomas.40 Contrary to the Lugano (and IWCLL) criteria, RECIL relies on unidimensional measurements (ie, the sum of long-axis diameters, SLD) of just up to 3 target lesions. This recommendation is based on the analysis of 47 828 imaging measurements from 2983 individual patients with adult and pediatric lymphoma enrolled in 10 multicenter clinical trials, which showed both a high correlation between unidimensional measurements and bidimensional areas and that the use of 3 instead of 6 target lesion does not have a relevant effect on the response category to which patients are assigned.40 Because of their ease of use, we therefore propose to use the RECIL criteria for response assessment in T-PLL, based on changes in lesion size on CT or magnetic resonance imaging (Table 5). [18F]FDG-PET-based response assessment, which is increasingly used in lymphoma, cannot be recommended at present, because there is practically no evidence with regard to the FDG avidity (ie, the glucose metabolism) of T-PLL. Other PET radiotracers, such as the CXCR4 tracer [68Ga]Ga-Pentixafor, which has shown promise in CLL, also require further evaluation.41
In line with the diagnostic workup of T-PLL, a number of different methods are used to detect remaining tumor cells when assessing the response to treatment. Immunophenotypic studies (flow cytometry or immunohistochemistry) are applied in addition to morphology. Furthermore, genetic analysis (TCR clonality testing, FISH, chromosome banding analysis) can be used to demonstrate the absence of a genetic aberration detected at diagnosis. These genetic tests show an analytical sensitivity that is roughly comparable to that of morphology and immunophenotyping in T-PLL, and can be applied complementarily to discriminate neoplastic from normal T cells.42 In addition, highly sensitive molecular tests (clone-specific real-time PCR or high-sensitive NGS analyses of TCR rearrangements) have been described for analysis of minimal residual disease (MRD) in T-PLL,43 and individual chromosomal breakpoints involving the TCL1 or MTCP1 locus could represent other targets for MRD assessment (by clone-specific real-time PCR or NGS) in T-PLL. With the current treatment options, the clinical relevance of low-level MRD in patients achieving CR remains unclear, and the value of standardized high-sensitive MRD testing needs to be assessed in forthcoming studies.
Best objective response rate (ORR) can be defined as a determined response with no additional improvement during at least 4 weeks of a continued or 8 weeks of an interval therapy. Given the acute nature of active T-PLL, an established response in peripheral blood (CR or CRi) requires confirmation by a bone marrow examination (aspirate and trephine), but does not necessarily need to be sustained for a certain period of time (in contrast to CLL). Other parameters are assessing the duration of responses in addition to ORR. Assessments to establish a CR, such as CT scans and bone marrow examination, should be performed after the patient has responded in the peripheral blood. Follow-up visits including blood count, chemistry, and physical examination after the end of any (continued or time-limited) therapy should be performed every 4 to 6 weeks.
With the advent of novel continuous treatment, stabilization of a prior rapidly progressing disease can be considered as beneficial for patients. We therefore propose inclusion of the term disease control rate which involves CR, CRi, partial remission (PR), and stable disease (SD). In the same way we would like to introduce the term disease control time.
CR
CR requires all the following criteria (Table 5).
1. Absence of lymphadenopathy
For previously enlarged lymph nodes (≥1.5 cm long-axis diameter), regression of the long-axis diameters to <1.0 cm (RECIL-2017) on CT or magnetic resonance imaging. Once response is established, further imaging should only be performed when disease progression is apparent by clinical examination or blood evaluation.
2. Absence of splenomegaly and hepatomegaly
Absence of splenomegaly or hepatomegaly by physical examination and by CT scan or ultrasound. In analogy to criteria for CLL a cutoff of 13 cm in craniocaudal length defines splenomegaly.12,44 At this time, there are no clear-cut criteria for hepatomegaly because of significant size differences among healthy individuals.
3. Absence of disease-related constitutional symptoms
4. Peripheral blood: Lymphocyte count <4 × 109/L
5. Clearance of phenotypic T-PLL cells in bone marrow (<5% of mononuclear cells)
A bone marrow aspirate and biopsy are required to confirm CR. They should be performed if clinical and laboratory results listed in Table 5 indicate that CR may be achieved. For CR, the cytological or histological evaluation of a technically adequate sample needs to document a bone marrow with T-PLL less than 5% of nucleated cells (assessed by immunohistochemistry or flow cytometry). In clinical trials aiming at evaluating deep remissions, flow cytometry and genetic analyses (FISH for gene rearrangements detected at diagnosis or PCR for clonality analysis of TCR gene rearrangement) may be applied to detect MRD. However, for the definition of CR, tests with a particularly high analytical sensitivity (<1%; see MRD) are not needed.
6. Absence of other specific site involvement
If previously involved, absence of pleural or peritoneal effusions, skin infiltrates, and involvement of central nervous system, or other extra-medullary sites.
7. Complete recovery of bone marrow function
Platelets ≥100 × 109/L (untransfused)
Hemoglobin ≥11.0 g/dL (untransfused, independent of Erythropoietin)
Neutrophils ≥1.5 × 109/L (independent of growth factor)
Complete remission with incomplete marrow recovery
These are patients achieving a CR with incomplete marrow recovery (CRi; fulfilling all criteria for CR in group A), but fail CR criteria in group B because of persistent anemia, thrombocytopenia, or neutropenia that are unrelated to T-PLL but, rather, to treatment toxicities. CRi has to be assessed prospectively in clinical trials to evaluate whether there is a prognostic significance when compared with regular CR and to PR.
PR
For a PR, at least 2 parameters of group A and 1 parameter of group B need to improve as specified if previously abnormal (Table 5). Only 1 parameter needs to improve if only 1 within each group was abnormal. Constitutional symptoms related to T-PLL should be noted because their presence for more than a month precludes a CR, but are irrespective to distinguish from other responses.
1. Decrease of lymphadenopathy
For previously enlarged lymph nodes (≥1.5 cm long-axis diameter), at least 30% regression in the SLD of up to 3 target lesions, but not CR (RECIL-2017).
2. Decrease of splenomegaly
Regression of splenomegaly by 50% or less in vertical length beyond normal (13 cm) from baseline compared with baseline.
3. <30 × 109/L circulating lymphocyte count in peripheral blood and decrease by 50% or more
4. Any result other than CR in bone marrow
Bone marrow aspirate and biopsy is performed if clinical and laboratory results indicate that a CR (PB and CT clearance) may have been achieved.
5. Partial recovery of bone marrow function of at least 1 of following parameters
Platelets ≥100 × 109/L (untransfused) or by 50% or more compared with baseline
Hemoglobin ≥11.0 g/dL or by 50% or more compared with baseline (untransfused, independent of Erythropoietin)
Neutrophils ≥1.5 × 109/L or by 50% or more compared with baseline (independent of growth factor)
SD
For SD, patients have not achieved a remission (CR or PR) and do not demonstrate progressive disease (PD). This state needs to be sustained for at least 3 months. Because a stabilization of a prior rapidly progressing disease over a certain period can be considered as beneficial for some patients, it should therefore not be called nonresponse or treatment failure. However, ORR only considers CR, CRi, and PR. If SD is included in the response measurements, it needs to be explicitly stated, as in the proposed term disease control rate and disease control time, both of which include CR, CRi, PR, and SD.
ORR
ORR is defined as the proportion of patients who have a CR, CRi, or PR, to therapy.
Disease control rate
Disease control rate is a composite of ORR and SD and is useful for continuous therapies that have tumorostatic effects.
PD
For PD, 1 or more parameters of group A are present. For group B, parameters (marrow impairment) need to be directly attributable to increased T-PLL marrow infiltration and unrelated to autoimmune cytopenias or treatment toxicities. For clarification, a bone marrow aspirate and biopsy may be required (Table 5). Progressive lymphadenopathy is indicated by a more than 20% increase in the SLD of up to 3 target lesions; for small lymph nodes measuring less than 1.5 cm after therapy, a minimum increase of 5 mm and a long-axis diameter of more than 1.5 cm, or appearance of a new lesion (RECIL-2017).
Progression-free survival
Progression-free survival (PFS) is defined as duration between the first treatment day and the time of first sign of progression or death from any cause.
Event-free survival
Event-free survival is defined as the interval between the first treatment day to the time of first sign of disease progression or start of new treatment or withdrawal from trial because of toxicities or death, whichever occurs first.
Time to next treatment
Time to next treatment is defined as interval between the first treatment day and the patient starting an alternative treatment of T-PLL.
OS
OS in a clinical trial is defined as the interval from the first treatment day to death from any cause.
Relapse
Relapse is defined as disease progression (PD; Table 5) after a CR, CRi, PR, or SD has been achieved and documented.
Refractory disease, treatment failure
Refractory disease and treatment failure are defined as disease progression (Table 5) without a previous response or disease control (CR, CRi, PR, or SD) having been achieved and documented.
MRD
MRD is defined by the detection of a small number of remaining T-PLL cells in PB or BM, using highly sensitive methods in a patient achieving CR or CRi. The clinical relevance of MRD assessment in T-PLL as well as the technical method remains to be established in forthcoming studies.
Current treatment of T-PLL
Very few trials have been conducted and published on T-PLL. Initial attempts with CHOP (cyclophosphamide, doxorubicin, vincristine, and prednisone) or CHOP-like regimens have been particularly discouraging.45 Table 6 summarizes the data of the most relevant clinical studies. The best first-line treatment to achieve a CR is IV alemtuzumab (anti-CD52) with a ORR higher than 90% and PFS between 8 and 11 months.4,46,47 Despite the high OR, patients will relapse, with a median duration of remission for responders of less than 2 years. For this reason, patients achieving a complete remission should be considered for consolidation therapy with an allogeneic stem cell transplant. About a third of patients may achieve long-term survival with this approach, although TRM and relapse rates remain high.48,49 In a prospective study from the European Society for Blood and Marrow Transplantation, patients who received conditioning with at least 6 Gy total body irradiation had a lower relapse rate.50,51 Autologous stem cell transplant is an option, particularly for patients without a donor, and has been shown to prolong PFS, but with no long-term survivors.48 There is no evidence to support alemtuzumab (or other) maintenance therapy.
Recommendation: IV alemtuzumab induction therapy for 10 to 12 weeks (to achieve best response), followed by consolidation with HSCT where feasible.
After relapse, the prognosis of T-PLL is dismal. Salvage regimens (Table 6) can achieve an ORR 50% to 76%, but only short duration and an OS of 6 to 9 months.7,15,47,52 There are preliminary in vitro and in vivo data to suggest that novel agents such as BCL2-inhibitors, HDAC inhibitors, and JAK3 inhibitors may have a therapeutic effect in TPLL.6,32,35,53,54 Clinical trials testing these novel therapeutic approaches are badly needed.
Discussion
The consensus criteria proposed here for diagnosis and treatment response evaluation in T-PLL are applicable for both daily clinical use and clinical studies. Although some of our recommendations are based on small to medium-sized clinical studies and observations on T-PLL itself, some have been extrapolated from other disease entities based on consensus evaluation. Historically, criteria for CLL, as defined by the IWCLL, have been used. However, CLL is very different from T-PLL and there is a need for the introduction of disease-specific criteria. The T-PLL- specific criteria presented here differ from the IWCLL criteria particularly in the following aspects:
The diagnosis of T-PLL relies on presence of clonal mature T cells with characteristic prolymphocytic morphology and distinct cytogenetics.
Instead of clinical staging according to Binet or Rai in CLL, we considered 2 disease states, inactive and active, as clinically more appropriate in T-PLL. At diagnosis, nearly all patients with T-PLL present with generalized disease as per highly elevated white blood cell count, splenomegaly, hepatomegaly, and generalized lymphadenopathy. So far, there are no reported observations or studies indicating that the number of areas of lymphoid tissues that are affected or whether hepatosplenomegaly might correlate with disease state or course or outcome. In comparison with other leukemias, especially acute leukemias (acute myeloid leukemia, acute lymphoblastic leukemia), staging criteria are not considered relevant and are therefore not established. Although the proposed phase-based distinction of T-PLL somewhat resembles the established system used in chronic myeloid leukemia, T-PLL does not typically show classical t-lymphoblasts-associated transformation. Nevertheless, an inactive (chronic) phase of T-PLL is usually followed by an active (acute) phase within 1 to 2 years. Neither cell-phenotypic changes (blastic transformation) nor other measurable changes other than altered disease kinetics and symptoms are detected on the transition into the active state of T-PLL. However, entering this active stage of disease requires treatment initiation (similar to Binet C or Rai III/IV stage in CLL).
Because T-PLL is clinically closer related to acute leukemias, we propose to confirm a response, such as a CR, with a bone marrow examination. However, as for acute leukemias, a clinical response that is confirmed in the bone marrow does not require to be maintained for a certain time.
We recommend the use of the recently proposed RECIL-2017 criteria for radiologic response assessment based on unidimensional changes (ie, SLD) of just up to 3 target lesions in CT or magnetic resonance imaging.40 RECIL-2017 are easier to use and appear more practical for both retrospective and prospective clinical studies and are nevertheless validated for lymphatic disease.
Ultimately, it will be critical and challenging to match the T-PLL consensus criteria as presented here in the consensus criteria to current and novel treatments. This will be an ongoing effort to optimize patient management and outcome in this rare leukemia. Future studies in T-PLL should not only evaluate the accuracy of these criteria but also try to establish the role of molecular assessments in prognostication, response prediction, and evaluation of depth of responses (minima residual disease) to better guide our treatments in this for the most part still-fatal disease.
Authorship
Contribution: P.B.S. organized the consensus meeting of the T-PLL International Study Group in May 2017 in Vienna, provided the first draft, and composed the last version of the manuscript; all authors of the manuscript are members of the T-PLL International Study Group; all authors discussed all recommendations until a consensus was reached, and all authors contributed equally to write and agree on the article.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Philipp B. Staber, Medical University of Vienna, Department of Medicine I, Division of Hematology & Hemostaseology, Währinger Gürtel 18-20, A-1090 Vienna, Austria; e-mail: philipp.staber@meduniwien.ac.at.