The pathophysiologic basis for reduced fitness in sickle cell disease (SCD) is multifactorial and characterized by impaired oxygen-carrying capacity, derangements in the cardiopulmonary response to exercise, and abnormal metabolic responses in working muscles. In this issue of Blood, Merlet et al1 demonstrated in a provocative and elegant study that exercise training led to muscle capillary growth in people with SCD.
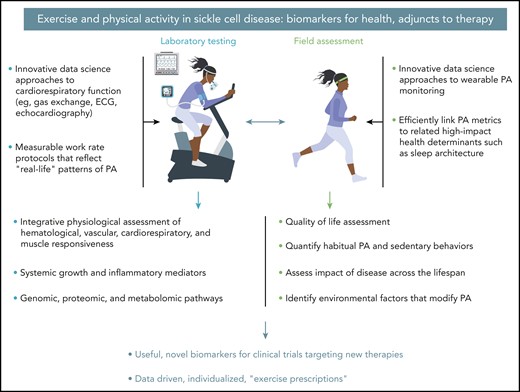
Leveraging innovative data science approaches to exercise laboratory testing and field assessments is a translational imperative in SCD that may lead to the development of novel biomarkers for new therapies and evidence-based exercise prescription. ECG, electrocardiogram.
Leveraging innovative data science approaches to exercise laboratory testing and field assessments is a translational imperative in SCD that may lead to the development of novel biomarkers for new therapies and evidence-based exercise prescription. ECG, electrocardiogram.
This is a remarkable observation because SCD is accompanied by increased inflammatory mediators,2 which (in other conditions of excessive inflammation such as type 2 diabetes) can inhibit regenerative functions like angiogenesis,3 and reduced habitual physical activity (PA),4 which activates “relentless deconditioning,” the well-known phenomenon of disuse atrophy.5 Although the observed biology of the training response in SCD was intriguing (no change in capillary morphology per se, rather, an increase in capillarization), the translational promise of the study is compelling. First, the training protocol itself was relatively mild (30 minutes per day of supervised cycle ergometry thrice weekly), and second, the investigators also found training-associated improvements of gas exchange measured noninvasively in formal cardiopulmonary exercise testing (CPET). By identifying a beneficial tissue-level mechanism of exercise training for SCD, Merlet et al demonstrated 1 potential pathway by which exercise training could in theory lead to disease-modifying benefits. They also set a data-driven stage for a transformation in the use of CPET and PA metrics as well as adjunctive therapies in SCD clinical trials in this era of revolutionary advances in SCD therapeutics (see figure).
Until recently, the roles of PA, exercise testing, and exercise prescriptions in SCD were poorly studied and controversial. Exercise can be a profound perturbation to cellular homeostasis, and the reduction in pH, increase in inflammatory mediators, and stress on the cardiorespiratory system6 associated with exercise testing or training were legitimate causes of concern in SCD individuals. Merlet et al point out in their article the growing body of literature demonstrating that exercise testing and exercise training can be safe and beneficial in this population. Our group has pioneered studies demonstrating the value of CPET biomarkers in children with SCD7 and is currently conducting a multicenter, National Institutes of Health (NIH)/National Heart, Lung, and Blood Institute–funded study to assess the impact of exercise intensity on inflammatory and safety end points in children with SCD (ClinicalTrials.gov Identifier: #NCT03653676). Collectively, these studies have been effective because the investigators adopted a scientifically rigorous approach to exercise: a biological state that all too often is defined ambiguously without an understanding of the physiological consequences of the intensity, frequency, and mode of the PA perturbation.
Progress in clinical applications of CPET and adjunctive therapeutic uses of PA (which, to be effective and to be studied, must be precisely defined and monitored in the real world) depend on the kind of studies published by Merlet and colleagues in Blood. However, providing scientific evidence and potential beneficial mechanisms of exercise even in the hostile internal biological milieu that unfortunately is present in SCD is not enough to improve the health of these patients at scale. The community of translational researchers must incorporate proven biological mechanisms and determine their value in clinical application, including impact on important clinical end points and patient-reported outcomes.
This has not been an easy task in the context of SCD. This is seen clearly when we compare 2 often-debilitating genetic diseases of children, cystic fibrosis (CF) and SCD. In the United States, there are ∼30 000 people with CF and 100 000 with SCD. A PubMed search of “exercise and clinical trials” revealed 127 publications about CF since 1966, but only 15 about SCD. It is not that the safety and role of exercise and PA are seen as unimportant in either CF or SCD. As part of the NIH/National Center for Advancing Translational Sciences (NCATS)–funded study project REACH (Revamping Exercise Assessment in Child Health), we conducted formal community engagement studios8 in collaboration with the NCATS Trial Innovation Network. Our community consultants (children and adolescents with CF or SCD and their primary caregivers) repeatedly noted how important participation in PA was, but how little guidance there was for both SCD and CF for safe and beneficial participation. Even in CF, in which Nixon et al9 demonstrated in the 1990s the clinical benefit of physical fitness, implementation of guidelines for CPET and exercise prescriptions has been painfully slow.
Now is the time for creation of a learning health systems10 approach to clinical application of exercise and PA metrics in SCD. Never has there been such great promise for breakthrough therapies in SCD. Advances in data science approaches to CPET and to wearable monitoring of PA, which can now evaluate additional critical components of health, such as sleep, will allow previously unimaginable real-life biomarkers of health. The NIH Common Fund–supported national consortium is beginning to enroll adults and children in the Molecular Transducers of Physical Activity (ClinicalTrials.gov Identifier: #NCT03960827), an unprecedented effort (focused on healthy individuals) that will provide the scientific community with data to elucidate the molecular pathways that link exercise to health.
The work of Merlet et al also addressed some of the challenges in translating more basic science observations to a real-world clinical framework. As noted, their training protocol was mild and feasible for the majority of the SCD adults who volunteered. They also provided evidence that increased muscle capillarization (observed in their biopsies) was accompanied by independent improvement in the gas exchange response (oxygen uptake and power output at key CPET stages) measured during submaximal rather than maximal exercise; thus, their testing and training protocols offer alternative and feasible models for clinical investigators concerned about exposing SCD patients to the inflammatory and physiological stress of maximal exercise. In sum, an approach to future studies is emerging that fully employs the minimally invasive, systemic, integrative physiologic data collected during CPET. It is hoped that the work of Merlet et al and other pioneering researchers will stimulate an international effort to improve the lives of people living with SCD using exercise biomarkers to gauge the effectiveness of new therapies, and to identify the role of regular exercise as an adjunctive therapy in and of itself.
Conflict-of-interest disclosure: The authors declare no competing financial interests.