Abstract
The development of brentuximab vedotin has opened a new era in the management of peripheral T-cell lymphomas (PTCLs). The improved outcomes with brentuximab vedotin (BV) in combination with cyclophosphamide, doxorubicin, and prednisone (BV-CHP) vs cyclophosphamide, doxorubicin, vincristine, and prednisone in the ECHELON-2 trial are practice changing for common nodal CD30+ PTCLs. Questions regarding the optimal cutoff of CD30 expression for BV-CHP therapy and the efficacy and safety of BV-CHP in less common subtypes of CD30+ PTCL subtypes await clarification.
Introduction
Peripheral T-cell lymphomas (PTCLs) are a heterogeneous group of lymphoid neoplasms with remarkable disparities in global incidence and prevalence.1 PTCLs represent ≤10% to 12% of non-Hodgkin lymphomas in the United States, although accurate prevalence rates in the community are unknown, especially for individual subtypes.2,3 Diagnostic accuracy is poor, with discordance rates of 35% to 40%4 and inconsistencies in the immunohistochemical panels used.5 Most PTCLs are aggressive, present with extensive disease, and have intermediate- or high-risk features.6-12 With the exception of anaplastic lymphoma kinase (ALK)+ anaplastic large cell lymphoma (ALCL), front-line cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP)-based regimens, with or without etoposide, result in 5-year overall survival (OS) and progression-free survival (PFS) ≤ 30%.6-12 Other front-line regimens have shown response rates, PFS, and OS comparable to CHOP in small single-arm13-16 or randomized phase 2 trials.17 Autologous stem cell transplant (ASCT) consolidation in fit age-eligible responders is routinely considered,18 but dropout rates for primary progression in intent-to-treat analyses are high (30-35%), and only a minority (10-26%) of patients receive ASCT in the community.19-24 The lack of novel agents to enhance front-line therapy has prevented progress.25
Frequency and patterns of CD30 expression in PTCL
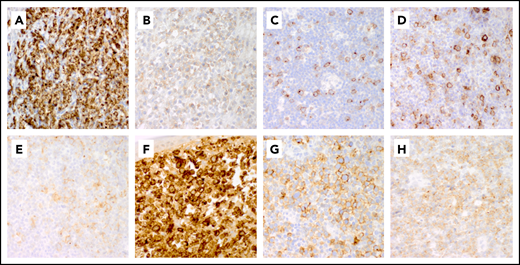
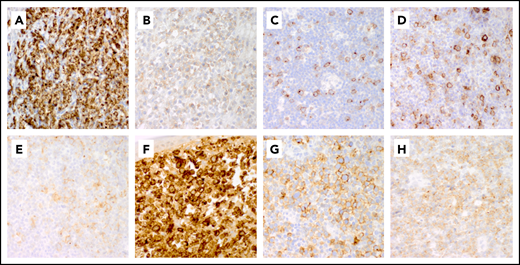
CD30 was identified as a protein expressed by Hodgkin Reed-Sternberg cells in Hodgkin lymphoma (HL) and by neoplastic T cells in ALCL.26-30 Among PTCLs, ALCLs show the most intense and uniform expression of CD30, regardless of ALK gene rearrangements.31 Partial and weaker expression are found in PTCL, not otherwise specified (PTCL-NOS; 58-64%), angioimmunoblastic lymphoma (AITL; 63-75%), enteropathy-associated T-cell lymphomas (EATLs; 0-100%), human T-cell leukemia virus, type 1–associated adult T-cell leukemia/lymphomas (ATLLs; 0-55%), and extranodal natural killer/T-cell lymphomas (36-47%).32-37 CD30 expression is absent in hepatosplenic and intestinal γ/δ PTCL, with rare positive cutaneous cases.38 CD30 is also variably expressed in mycosis fungoides and Sézary syndrome, particularly with large cell transformation.39-41 In PTCL, the levels of CD30 messenger RNA and protein are closely related.36 Immunohistochemistry on formalin-fixed paraffin-embedded tissue with the monoclonal antibody BerH2 is routinely used to detect CD30 expression, with a “membrane and dot/cytoplasm” staining pattern (Figure 1). However, there is a lack of consensus on positive cutoffs for CD30 expression. Studies have used the percentage of positive cells, staining intensity, or both to determine positive cutoff values (≥1%, ≥10%, or 20-25%) (Table 1), but the clinical impact of different criteria is unclear.
Representative patterns of CD30 expression in non-ALCL T-cell lymphomas. (A-C) Variable expression patterns of CD30 in PTCL-NOS. Strong uniform CD30 expression in PTCL-NOS (A), weak CD30 expression in PTCL-NOS (B), and partial CD30 expression in PTCL-NOS (C). (D-E) CD30 expression in AITL. (D) CD30 is expressed in large immunoblasts and in smaller neoplastic cells in AITL. (E) Weak CD30 expression in AITL. (F) Transformed mycosis fungoides with strong diffuse CD30 expression. (G-H) Patterns of expression of CD30 in ENKTL. Moderate partial CD30 expression in ENKTL (G) and diffuse weak CD30 expression in ENKTL (H). Original magnification ×50 for all panels.
Representative patterns of CD30 expression in non-ALCL T-cell lymphomas. (A-C) Variable expression patterns of CD30 in PTCL-NOS. Strong uniform CD30 expression in PTCL-NOS (A), weak CD30 expression in PTCL-NOS (B), and partial CD30 expression in PTCL-NOS (C). (D-E) CD30 expression in AITL. (D) CD30 is expressed in large immunoblasts and in smaller neoplastic cells in AITL. (E) Weak CD30 expression in AITL. (F) Transformed mycosis fungoides with strong diffuse CD30 expression. (G-H) Patterns of expression of CD30 in ENKTL. Moderate partial CD30 expression in ENKTL (G) and diffuse weak CD30 expression in ENKTL (H). Original magnification ×50 for all panels.
Development of brentuximab vedotin in CD30+ lymphomas
Clinical trials with an unconjugated CD30-targeting monoclonal antibody (cAC10, SGN-30) demonstrated safety in patients with CD30+ lymphomas but low response rates.42,43 To increase efficacy, SGN-30 was “armed” with a monomethyl auristatin E toxin payload, producing a potent anti-CD30 antibody drug conjugate (SGN-35).44,45 SGN-35 (brentuximab vedotin [BV]) showed high potency and selectivity against HL and ALCL in vitro and in vivo. A phase 1 trial of BV in 45 patients with relapsed or refractory (R/R) CD30+ hematologic malignancies, primarily (93%; n = 42) HL, identified the maximum tolerated dose as 1.8 mg/kg every 3 weeks.46 Toxicities included fatigue (36%), fever (33%), and diarrhea, nausea, neutropenia, and peripheral sensory neuropathy (PSN) (22% each). The objective response rate (ORR) across all dose levels was 38% (n = 17/45), with a complete response (CR) rate of 24% (n = 11/45). Among 12 patients treated at the maximum tolerated dose, the ORR was 50% (n = 6), with 33% achieving a CR (n = 4), leading to 2 pivotal phase 2 trials in ALCL and HL. Pro and colleagues47 studied BV, 1.8 mg/kg every 3 weeks up to 16 doses, in 53 patients with R/R ALCL. ORR was 86%, with 57% CR. The median duration of response was 12.6 months, with median PFS of 13.3 months and 1-year OS of 70%. Twenty-eight patients (53%) received stem cell transplant (SCT) following BV (22 autologous HCT; 6 allogeneic HCT). Adverse events (AEs) mirrored the phase 1 experience, with PSN (41%), nausea (40%), fatigue (38%), and fever (34%) being the most common. AEs ≥ grade 3 were mostly hematological (21% neutropenia, 14% thrombocytopenia, 7% anemia) or neurological (12% PSN). A recent update48 shows that, of the 38 patients in CR, 16 remained in remission after a median follow-up of 71.4 months. The 5-year OS was 60%. For patients with CR as best response, the 5-year estimated PFS and OS were 57% and 79%, respectively. Patients who had a negative positron emission tomography/computed tomography after cycle 4 (iPET4) did particularly well, with an estimated 5-year PFS of 64% vs 16% for patients with a positive iPET4. The 16 patients who underwent post-BV SCT had a 5-year PFS of 69% and 5-year OS of 75%, compared with 48% and 81%, respectively, in the 22 patients who did not. These findings suggest that some patients, particularly early responders (ie, negative iPET4), can have durable remissions without SCT.
BV in R/R CD30 PTCL
To explore the benefit of targeting CD30 in lymphomas other than HL and ALCL, Horwitz and colleagues49 assessed the efficacy of BV in R/R CD30+ PTCL, defined as any detectable CD30 by an institutional laboratory. Of 35 enrolled patients, 22 (63%) had PTCL-NOS, and 13 (37%) had AITL. The ORR for 34 evaluable patients was 41% (CR 24%); in AITL, the ORR was numerically higher at 54% (CR 38%) than in PTCL-NOS (ORR, 33%; CR, 14%). Median duration of response was 5.5 months for AITL patients and 7.6 months in PTCL-NOS. Median PFS for all patients was only 2.6 months (6.7 months for AITL; 1.6 months for PTCL-NOS). However, 10 patients (29%) had responses lasting >6 months. Treatment was discontinued for disease progression in 57% and for AEs in 20%, most frequently PSN (6%). CD30 expression did not correlate with efficacy; responses were seen in patients with ≤15% CD30 (ORR, 64%). Notably, 6 (17%) of the enrolled patients had undetectable CD30 expression by central review.
BV in the front-line setting
Two strategies to combine BV with CHOP in untreated CD30+ PTCL, defined as ≥1% CD30-expressing neoplastic cells, were tested.50 In a sequential approach, 2 doses of BV (1.8 mg/kg every 3 weeks × 2) were given prior to 6 cycles of CHOP (BV>CHOP); in a concurrent approach, BV (1.8 mg/kg) replaced vincristine, and BV-CHP (BV in combination with cyclophosphamide, doxorubicin, and prednisone) was given for a total of 6 cycles every 3 weeks. Patients could receive single-agent BV for 8 to 10 additional doses if they had achieved at least a partial response after BV-CHP. Thirty-nine treatment-naive patients were enrolled (13 sequential, 26 concurrent). Median age was 57 years (range, 21-82). The most common subtype was systemic ALCL (sALCL; 84%, n = 6 ALK+; n = 26 ALK−), whereas 7 had non-ALCL PTCL. CD30 expression ranged from 20% to 100%. In the BV>CHOP cohort, all patients received the initial 2 doses of BV, and 12 of 13 went on to BV maintenance, with an ORR of 85% (CR, 62%). In the BV-CHP cohort, ORR was 100%, with 88% CR, and 21 of 23 received maintenance BV. Toxicities were similar in both cohorts, including PSN (69% and 77%); however, diarrhea and alopecia were more common with BV-CHP, and vomiting was more common with BV>CHOP. For BV>CHOP, estimated 1-year PFS and OS rates were 77% and 85%, respectively. For BV-CHP, the estimated 1-year PFS and OS rates were 71% and 88%, respectively. No patient received SCT. Updated long-term outcomes (median follow-up, 59.6 months) for the BV-CHP cohort51 show 5-year PFS and OS rates for all patients of 52% and 80%, respectively, with no death or progression beyond 35 months. When analyzed by subtype, outcomes for sALCL appeared comparable to non-ALCL subtypes, with estimated 5-year OS of 79% and 83%, respectively. ALK+ sALCL patients fared most favorably (5-year PFS, 100% vs 38%). Of the initial 19 patients (73%) with PSN, 18 (95%) reported resolution or improvement of symptoms.
ECHELON-2
The benefit of integrating BV in the front-line therapy of a subset of CD30+ PTCL was conclusively demonstrated by the ECHELON‐2 study, a phase 3 randomized double‐blind double‐dummy placebo-controlled trial of BV-CHP vs CHOP in adults with newly diagnosed CD30+ PTCL, defined as ≥10% CD30+ tumor cells by local immunohistochemistry.52 Patients were stratified by International Prognostic Index (IPI) and histology (ALK+ ALCL vs other) and randomized 1:1 to receive BV-CHP or CHOP, with placebo (dummy) instead of vincristine or BV, respectively, for 6 to 8 cycles, every 21 days. The primary efficacy end point was PFS, by independent review. Secondary end points included ORR, CR rate, and OS. The study randomized 452 patients (ALCL, 70% [22% ALK+ with IPI ≥ 2]; PTCL-NOS, 16%; AITL, 12%; ATLL, <2%; EATL, <1%), 449 of whom were treated (223 with BV-CHP, 226 with CHOP). Median age for both arms was 58 years (range, 18-85). Most patients had stage III/IV disease (81%) and IPI ≥ 2 (77.5%).
With a median follow-up of 36.2 months, BV-CHP was superior to CHOP for the primary end point (PFS, 48.2 months vs 20.8 months; hazard ratio, 0.71), primarily as the result of more treatment failures with CHOP, as well as for all secondary end points, including CR rate (68% vs 56%), ORR (83% vs 72%), and OS (hazard ratio, 0.66; median OS was not reached in either arm). BV-CHP resulted in a 29% and 34% reduction in the risk of progression and death, respectively. The spectrum of BV-specific toxicities with BV-CHP mirrored that previously observed, with no new safety signals. Incidences and patterns of sensory peripheral neuropathy were similar with BV-CHP and CHOP, with 50% of the patients having resolution and 12% experiencing partial improvement of sensory peripheral neuropathy at last assessment and median time to resolution or improvement of 4 months (range, 0-45).
Impact for practice
Thanks to improved PFS and OS compared with CHOP, BV-CHP is the first front-line regimen approved by the US Food and Drug Administration for PTCL. Because ECHELON-2 enrollment had a predefined target of 75% sALCL, the superiority of BV-CHP was primarily driven by this subtype, supporting BV’s addition to front-line chemotherapy as a new standard for these patients, including those with IPI ≥ 2 ALK+ sALCL. What about other regimens? In a subset analysis of German clinical trials,53 the addition of etoposide to CHOP in PTCL patients older than 60 years of age led to excessive toxicity and no efficacy benefit compared with CHOP, an unmet need that BV-CHP is well positioned to address. Although median age was 58 years (range, 18-85), ECHELON-2 enrolled 139 patients ≥65 years of age. Safety was comparable, and the analysis of key subgroups did not show that PFS and OS improvements were limited to younger (<65 years) patients. For patients ≤60 years (and with normal lactate dehydrogenase), the benefit of etoposide+CHOP vs CHOP in the German studies was only seen for PFS (and retrospectively), a less robust benchmark. In our opinion, this makes BV-CHP a new front-line standard for all sALCL patients. Whether BV-CHP eliminates the need for ASCT in ALK− ALCL and high-risk ALK+ ALCL is not clear.
The role of BV-CHP in PTCLs other than ALCL and the optimal CD30 expression cutoff for patient selection remain to be determined. Although non-ALCL (PTCL-NOS, n = 72; AITL, n = 54) outcomes in ECHELON-2 were consistent with the overall study results, the study was not powered to show superiority in these subtypes, and additional data are needed. Because the US Food and Drug Administration label supports the use of BV-CHP in CD30-expressing PTCLs, irrespective of subtype, BV-CHP can be used in non-ALCL PTCL patients who match ECHELON-2 eligibility criteria, an approach that we support. Currently, there are few data for the front-line use of BV-CHP in CD30+ PTCL subtypes not included in ECHELON-2. The optimal level of CD30 expression to benefit from BV-CHP in PTCL is unknown. Nine patients on the ECHELON-2 BV-CHP arm had <10% CD30+ after central review. Of those, 7 (78%) responded and 5 (56%) achieved a CR, suggesting that a ≥10% cutoff may not be necessary. Data from past and ongoing trials of single‐agent BV in CD30+ lymphomas show objective responses and CRs with <10% CD30 expression, including patients with no detectable CD30.54-57 Conversely, low response rates to BV in other CD30+ lymphomas suggest that CD30 expression is not sufficient for antitumor response.58 It is unknown whether responses to single-agent BV in patients with very low CD30 expression might translate into superiority of BV-CHP over CHOP.
Conclusions
BV has opened new vistas in PTCL, and the positive results of ECHELON-2 are practice changing. Questions regarding the optimal CD30 expression level, the activity of BV-CHP in less common CD30+ PTCLs, and the role of ASCT after BV-CHP await clarification. Postapproval surveillance for long-term safety is important. New BV-based combinations are being explored (Table 1).
Acknowledgment
The histopathology panel for the ATLL image in the visual abstract is courtesy of A. A. Gru, University of Virginia.
Authorship
Contribution: P.P., S.K.B., and J.Z.G. wrote and edited the manuscript; and J.Z.G. procured original pathology images and produced Figure 1.
Conflict-of-interest disclosure: P.P. and S.K.B. have received research funding from Seattle Genetics. J.Z.G. declares no competing financial interests.
Correspondence: Pierluigi Porcu, Division of Hematologic Malignancies and Hematopoietic Stem Cell Transplantation, Department of Medical Oncology and Sidney Kimmel Cancer Center, 834 Chestnut St, Suite 320, Philadelphia, PA 19107; e-mail: pierluigi.porcu@jefferson.edu.