Introduction: Hereditary hemorrhagic telangiectasia (HHT, Osler-Weber-Rendu disease) is a rare hereditary multisystem vascular disease of disordered angiogenesis. Pathologic elevations in vascular endothelial growth factor (VEGF) result in fragile, abnormal vessels in nasal and gastrointestinal (GI) mucosa causing recurrent epistaxis, chronic GI bleeding, and anemia that is often transfusion-dependent. Bevacizumab is a monoclonal IgG1 antibody that neutralizes circulating VEGF and is a potential targeted anti-angiogenic treatment in HHT, which has no FDA-approved therapy. Data for use of intravenous (IV) bevacizumab in HHT is currently limited to case reports and small single-center retrospective case series. The International HHT Intravenous Bevacizumab Investigative Team study of Bleeding (InHIBIT-Bleed) is an international collaboration of HHT Centers seeking to more definitively describe the safety and effectiveness of systemic bevacizumab to treat chronic bleeding and anemia in HHT.
Methods: The following data was retrospectively collected at each participating center for all HHT patients treated for at least 3 months with off-label systemic bevacizumab for chronic bleeding: demographics, baseline HHT characteristics, epistaxis severity score (ESS, a validated 10-point scale to evaluate epistaxis in HHT), bevacizumab dosing, treatment-emergent adverse events (TEAEs), hemoglobin (Hgb), red blood cell (RBC) transfusions, and IV iron infusions. Hgb and ESS at baseline were compared with post-bevacizumab values at 3, 6, 9, and 12 months with the paired t-test. RBC transfusion and iron infusion requirements before and after bevacizumab treatment were compared with the Wilcoxon signed rank test.
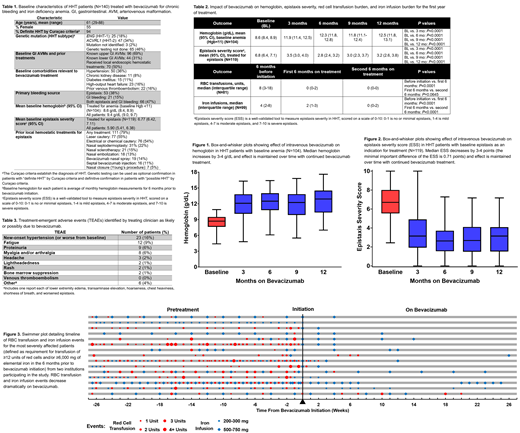
Results: 140 HHT patients were treated with IV bevacizumab for chronic bleeding for a median of 23 (range, 3-96) months with a median of 12 (range, 2-74) total infusions. Bevacizumab was dosed at 5 mg/kg every 2 weeks for the first 2 months, then monthly for 4 months; following this, maintenance dosing practices varied depending on center. Baseline patient characteristics are given in Table 1. After bevacizumab initiation, patients with baseline anemia (Hgb<11, N=104) had an increase in mean Hgb from 8.6 g/dL to 11.9 g/dL at 3 months (P<0.0001) up to 12.5 g/dL at 12 months (P<0.0001) (Table 2, Figure 1). For patients with epistaxis as an indication for treatment (N=119), mean ESS dropped from 6.8 at baseline to ≤3.5 on treatment (P<0.0001) (Table 2, Figure 2). In patients requiring RBC transfusion prior to bevacizumab (N=81), transfusion requirements decreased from a median of 8 units in the 6 months before treatment to a median of 0 units in the first 6 months after treatment (P<0.0001); similarly, in patients requiring iron infusion prior to bevacizumab (N=99), requirements decreased from a median of 4 infusions to 2 infusions (P<0.0001) (Table 2). RBC transfusion and iron infusion requirements continued to improve with ongoing bevacizumab treatment (Table 2) and improvement occurred regardless of baseline bleeding severity; Figure 3 illustrates the dramatic reduction in RBC transfusion and iron infusion requirements in a severe patient sub-cohort. Overall, compared with pretreatment, RBC transfusion and iron infusion requirements decreased by 86% and 66%, respectively, on bevacizumab. On subgroup analysis by underlying mutation (ENG vs. ACVRL1), there were no significant differences in baseline parameters or bevacizumab response. Bevacizumab was well-tolerated; TEAEs felt to be likely or possibly related to bevacizumab occurred in 36% of patients (Table 3), with new or worsened hypertension (16%) and fatigue (9%) most common. No patient developed venous thromboembolism on bevacizumab. Only 5 patients (4%) discontinued bevacizumab for TEAEs and only 3 patients (2%) discontinued bevacizumab for lack of effectiveness.
Conclusions: We present results of IV bevacizumab treatment of chronic bleeding and anemia in a large cohort of 140 HHT patients, demonstrating that bevacizumab was highly effective, with an improvement in mean Hgb of 3.9 g/dL, dramatic reduction in ESS, and decrease in RBC transfusion and iron infusion requirements by 86% and 66%, respectively. Effectiveness was maintained over time. Safety analysis included a total of 194 patient-years of IV bevacizumab administration to HHT patients and had low TEAE rates with only 4% discontinuing treatment due to TEAEs.
Al-Samkari:Agios: Consultancy, Research Funding; Moderna: Consultancy; Dova: Consultancy, Research Funding. Kuter:Caremark: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Dova: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Zafgen: Consultancy, Honoraria; Platelet Disorder Support Association: Consultancy, Honoraria; Shinogi: Consultancy, Honoraria; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; Agios: Consultancy, Honoraria, Research Funding; Platelet Disorder Support Association: Consultancy, Honoraria; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Caremark: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Merck Sharp Dohme: Consultancy, Honoraria; Merck Sharp Dohme: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Argenx: Consultancy, Honoraria, Research Funding; Bristol Myers Squibb (BMS): Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Kezar: Research Funding; Shire: Consultancy, Honoraria; Kezar: Research Funding; Amgen: Consultancy, Honoraria, Research Funding; Agios: Consultancy, Honoraria, Research Funding; Principia: Consultancy, Honoraria, Research Funding; Zafgen: Consultancy, Honoraria; Alnylam: Consultancy, Honoraria, Research Funding; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Actelion (Syntimmune): Consultancy, Honoraria, Research Funding; Principia: Consultancy, Honoraria, Research Funding; Protalex: Consultancy, Honoraria, Research Funding; Protalix: Consultancy, Honoraria; Protalix: Consultancy, Honoraria; Rigel: Consultancy, Honoraria, Research Funding; Rigel: Consultancy, Honoraria, Research Funding; Sanofi: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria; UCB: Consultancy, Honoraria; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; Up-to-Date: Consultancy, Honoraria, Patents & Royalties: 3 Up-to-Date chapters; UCB: Consultancy, Honoraria; Kyowa-Kirin: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Genzyme: Consultancy, Honoraria; Protalex: Consultancy, Honoraria, Research Funding; Argenx: Consultancy, Honoraria, Research Funding; Pfizer: Consultancy, Honoraria; Shinogi: Consultancy, Honoraria; Takeda (Bioverativ): Consultancy, Honoraria, Research Funding; Shire: Consultancy, Honoraria; Alnylam: Consultancy, Honoraria, Research Funding; Dova: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Momenta: Consultancy, Honoraria; Momenta: Consultancy, Honoraria.
The presentation will discuss off-label use of systemic bevacizumab for the treatment of chronic bleeding and anemia in hereditary hemorrhagic telangiectasia.
Author notes
Asterisk with author names denotes non-ASH members.