Background: Kell and Rhesus (Rh) maternal red blood cell (RBC) alloimmunization are the most common causes of severe fetal haemolytic disease. Widespread use of anti-D immune globulin has dramatically reduced the incidence of Rh(D) alloimmunization, leaving K alloimmunization responsible for a significant proportion of cases of fetal anemia requiring intrauterine transfusion ( ). K antigens are expressed on fetal erythrocytes at 10-11 weeks and k antigens at 6-7 weeks gestation (Tovey et al, 1986). In alloimmunized women, erythroid specific antibodies traverse the placenta, causing immune destruction of fetal erythroid cells leading to progressive haemolytic anaemia. The mechanism of fetal anaemia in K alloimmunization differs somewhat from that in Rh-D, in that anti-K antibodies cause suppression of fetal erythropoiesis (Gariod et al, 2004; Weiner et al, 1996). Whilst IUT of RBCs has improved fetal and neonatal survival, important information such as the critical anti-K titres to guide appropriate timing and frequency of IUT, is somewhat conflicting. Currently anti-K alloimmunized pregnancies do not have a standardized protocol for titer monitoring throughout pregnancy and, once alloimmunized, patients are usually referred to a regional fetal center for close ultrasound (US) surveillance. Our primary objective is to determine from a retrospective analysis of our population whether there is a critical anti-K titer that should trigger intensive US monitoring or intervention and to investigate the rate of progression of fetal anemia following IUTs.
Methods: This is a retrospective single-center study at Mount Sinai Hospital (MSH), Toronto, Canada, of all pregnant patients with anti-K as the primary alloimmunizing antibody, between 1991 and 2018. MSH is the largest fetal medicine center in Canada and the largest referral center for IUTs. Ethical approval was granted by the Research Ethics Board (REB # 12-0113-C). Data were obtained from a database of patient medical records, US reports and the transfusion medicine laboratory, including maternal demographics, pregnancy history, presence of other alloantibodies and hemoglobin concentration before and after all IUTs. Neonatal outcomes included survival, mode of delivery, gestational age (GA) at delivery, birth weight and need for neonatal exchange transfusion, phototherapy or IVIG. Data were analyzed using GraphPad Prism 6 and linear correlations are expressed as a p-value.
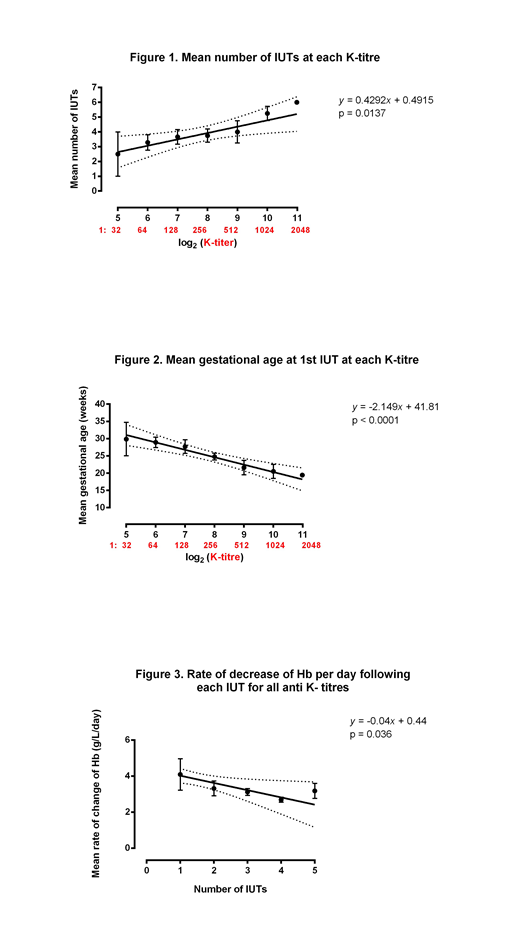
Results: Thirty-eight women underwent 163 IUTs in 44 pregnancies where K was the predominant antibody. Two patients in whom anti-K was a secondary antibody were excluded. In 5 of these pregnancies, 2 had a total of three alloantibodies and 5 had 2 alloantibodies each. The median maternal age was 31 (29 - 35) years. Four women had a history of intrauterine fetal death (IUFD) and 9 of neonatal haemolytic disease. The median GA at 1st IUT was 24.2 weeks (14.9-34.7), and there was a median of 4 IUTs per patient. There were seven cases of hydrops fetalis. The number of IUTs a patient received throughout pregnancy was correlated directly with the anti-K titer (Figure 1). Every 4-fold dilution resulted in a further increase in the IUT number by 2.2 above the mean of 2.5 at a titer of 1:32 (p=0.0137). Figure 2 illustrates the correlation between the GA at 1st IUT and antibody titer. IUTs were required at earlier GAs if the titers were higher. Following a 1:32 titer, every 2-fold titer increase reduced the mean gestational age at 1st IUT by 2 weeks (p<0.0001). No pregnancy required an intervention with an anti-K titer < 1:32. The median fetal Hb at the first IUT was 48 (8-91) g/L with an average daily decrease of hemoglobin of 4.7 g/L/day between the first and second IUT (Figure 3), which is similar to the 4.1 g/L/day previously reported in Rh(D) alloimmunization. The median GA was 37.1 (24-39.7) weeks at delivery with median birth weight centile of 59.5%; 8.9% of neonates required a blood transfusion and 24.4% required phototherapy. There were four IUFDs and one neonatal .
Conclusion: Our data support a critical anti-K titer of 1:32 and support a role for anti-K titer monitoring as a predictor of disease severity, counselling women appropriately and establishing a balance between paternal K antigen typing, US middle cerebral artery peak systolic velocity monitoring of the fetus and IUTs.
Garbowski:Vifor Pharma: Consultancy; Imara: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.