We have previously reported that cytarabine/daunorubicin as well cytarabine/idarubicin can be safely combined with crenolanib at the full monotherapy dose (100mg TID) and administered throughout induction, consolidation and maintenance in newly diagnosed FLT3-mutated AML (Walter et al., EHA 2018). We hypothesize that crenolanib plasma levels might correlate with long term outcome in these patients.
Methods: Twenty-seven newly diagnosed AML patients with FLT3 mutations, aged 18-60 years old enrolled in a phase II study of crenolanib combined with chemotherapy (NCT02283177) were included in this analysis. Patients received 7+3 induction with cytarabine 100 mg/m2 for 7 days and either daunorubicin 90 mg/m2 (n=16) or idarubicin 12 mg/m2 (n=11) for 3 days. Crenolanib 100 mg TID was administered continuously starting 24-48 hours after induction until 72 hours prior to the next chemotherapy cycle. Consolidation could consist of up to four cycles of high-dose cytarabine (HiDAC): 3 g/m2 for < 60 years and 1 g/m2 for 60 years) q12 hours on days 1, 3, and 5 with crenolanib starting 24 hours after the final HiDAC dose in each cycle. Eligible patients proceeded to allogeneic hematopoietic stem cell transplant (HSCT). Maintenance crenolanib at 100mg TID was started after HiDAC or 30-90 days after HSCT for up to 12 cycles.
Crenolanib levels (total and free) were measured 2-4 hours following the first dose of crenolanib and after two weeks of continuous crenolanib administration. Crenolanib levels were also measured on first day of crenolanib treatment following HiDAC consolidation. Free crenolanib was measured by rapid equilibrium dialysis. Durability of clinical remissions were documented by routine follow up every 3-4 months (as per institutional practice).
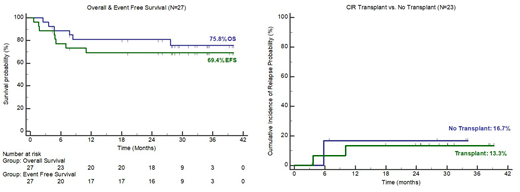
Results: As of July 2019, all patients have completed all protocol therapy (one patient has continued crenolanib maintenance for 25 months per patient request). Twenty-three of 27 patients (85%) achieved complete remission, all of whom required only 1 cycle of induction chemotherapy. Nineteen of 27 patients remain alive and free of disease with a median follow-up of 29.3 months (Figure 1). Three relapses have occurred, all within the first year of treatment and no relapses have occurred in patients who completed at least 1 cycle of crenolanib maintenance. Seven patients received HiDAC consolidation along with crenolanib but did not receive HSCT. Six of these patients remain in long term remission. While the number of patients is small, we observed similar overall survival and cumulative incidence of relapse in patients who underwent HSCT as compared to those who did not (Figure2).
Pharmacokinetic studies demonstrate that despite increases in acute phase reactants and AGP levels, both total and free crenolanib rapidly achieve sufficient levels to provide the continuous inhibition required to eradicate FLT3-ITD, FLT3-D835, and other FLT3 mutations including FLT3-N841, A680, V592, V491, and D839. Total levels of crenolanib exceeded 500 nM at steady state, and the majority of patients showed clearance of FLT3 mutations after combination therapy.
Summary/Conclusion: These pharmacokinetic data show that crenolanib when given with chemotherapy can achieve sufficient levels to inhibit multiple FLT3 mutations, despite rises in FLT3 ligand and acute phase reactants. These clinical data suggest that this combination might improve outcomes in younger patients with newly diagnosed FLT3-mutant AML. With 29 months median follow up, median overall survival (OS), event-free survival (EFS), and cumulative incidence of relapse (CIR) have not been reached. No relapses have been observed in patients after completing crenolanib maintenance. A phase III randomized multicenter trial has been initiated to compare the efficacy of crenolanib versus midostaurin combined with standard chemotherapy for newly diagnosed patients with FLT3-mutant AML (NCT03258931).
Goldberg:Abbvie: Research Funding; ADC Therapeutics: Research Funding; American Society of Clinical Oncology: Research Funding; American Society of Hematology: Research Funding; Arog Pharmaceuticals: Research Funding; Daiichi-Sankyo: Consultancy, Research Funding; Pfizer: Research Funding; DAVA Oncology: Honoraria; Abbvie: Consultancy; Celgene: Consultancy. Coombs:Abbvie: Consultancy; Covance: Consultancy; Dedham Group: Consultancy; H3 Biomedicine: Honoraria; Cowen & Co.: Consultancy; Octopharma: Honoraria; Pharmacyclics: Honoraria; Loxo: Honoraria; Medscape: Honoraria. Wang:Kite: Other: Advisory role; Abbvie: Other: Advisory role; celyad: Other: Advisory role; Astellas: Other: Advisory role, Speakers Bureau; Jazz: Other: Advisory role; Pfizer: Other: Advisory role, Speakers Bureau; Stemline: Other: Advisory role, Speakers Bureau; Daiichi: Other: Advisory role; Amgen: Other: Advisory role; Agios: Other: Advisory role. Walter:Agios: Consultancy; Amgen: Consultancy; Amphivena Therapeutics: Consultancy, Equity Ownership; Aptevo Therapeutics: Consultancy, Research Funding; Argenx BVBA: Consultancy; Astellas: Consultancy; BioLineRx: Consultancy; BiVictriX: Consultancy; Boehringer Ingelheim: Consultancy; Boston Biomedical: Consultancy; Covagen: Consultancy; Daiichi Sankyo: Consultancy; Jazz Pharmaceuticals: Consultancy; Kite Pharma: Consultancy; New Link Genetics: Consultancy; Pfizer: Consultancy, Research Funding; Race Oncology: Consultancy; Seattle Genetics: Research Funding. Messahel:Arog Pharmaceuticals: Employment. Stone:Astellas: Consultancy; Biolinerx: Consultancy; Arog: Consultancy, Research Funding; Trovagene: Consultancy; Macrogenics: Consultancy; Argenix: Other: DSMB; Argenix: Other: DSMB; Otsuka: Consultancy; Takeda: Other: DSMB; Trovagene: Consultancy; Biolinerx: Consultancy; Daiichi-Sankyo: Consultancy; Celgene: Consultancy, Other: DSMB; Pfizer: Consultancy; Jazz: Consultancy; Roche: Consultancy; Actinium: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Abbvie: Consultancy, Research Funding; Arog: Consultancy, Research Funding; Stemline: Consultancy; Biolinerx: Consultancy; Pfizer: Consultancy; Astra-Zeneca: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Actinium: Membership on an entity's Board of Directors or advisory committees; Biosight: Consultancy; Arog: Consultancy, Research Funding; Otsuka: Consultancy; Abbvie: Consultancy, Research Funding; Astra-Zeneca: Consultancy; Roche: Consultancy; Argenix: Other: DSMB; Macrogenics: Consultancy; Amgen: Membership on an entity's Board of Directors or advisory committees; Jazz: Consultancy; Biosight: Consultancy; Otsuka: Consultancy; Agios: Consultancy, Research Funding; Agios: Consultancy, Research Funding; Novartis: Consultancy, Research Funding; Takeda: Other: DSMB; Astellas: Consultancy; Agios: Consultancy, Research Funding; Daiichi-Sankyo: Consultancy; Trovagene: Consultancy; Celgene: Consultancy, Other: DSMB; Stemline: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.