Introduction
In Asians, NUDT15 genetic variants have been reported to be associated with low tolerance to 6-mercaptopurine (6-MP). However, variation in 6-MP tolerance is still observed among patients without the NUDT15 variants. Some patients required high doses of 6-MP to obtain treatment effect. We performed a genome-wide association study (GWAS) in Japanese to confirm reported associations as well as to identify additional genetic loci associated with variation of 6-MP tolerance in childhood acute lymphoblastic leukemia (ALL) patients.
Methods
We included ALL patients enrolled into a Tokyo Children's Cancer Study Group (TCCSG) trial who started maintenance therapy by normal protocol dose (30-50 mg/m2/day). Whole genome single nucleotide polymorphism (SNP) microarray genotyping was performed followed by whole genome SNP imputation (Japanese reference genome). Data for over six million SNPs were available for analysis. Association analysis was performed adjusting for age at diagnosis and considering average 6-MP dose during 168 days from maintenance therapy initiation as the outcome.
Results
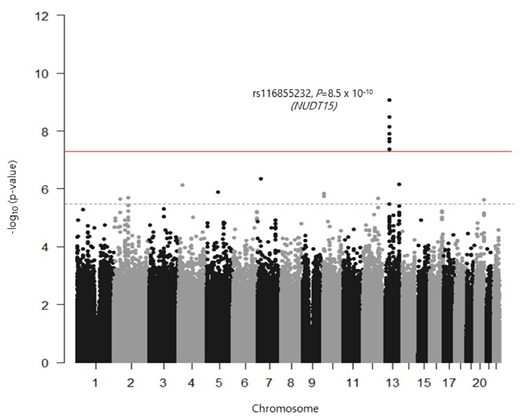
A total of 224 patients were included in the analysis. The median age at diagnosis was 4.7 (range = 0.9-15.7) years old and boys comprised 56.3%. The average 6-MP dose during the 168 days of maintenance therapy was 41.1 (range = 13.3-78.5) mg/m2/day. Variants representing 10 genomic regions showed potential association (P < 5 x 10-6) with average 6-MP dose, in which one locus was genome-wide significant (13q14.2, rs116855232). Variant rs116855232 is located in the previously identified NUDT15 gene and represents the strongest association with average 6-MP dose within our Japanese study (β = -11.45, P = 8.5 x 10-10). In contrast, the TPMT locus, which is predictive of 6-MP intolerance in populations of European ancestry, was not associated with 6-MP dose in Japanese. Among the suggestive loci requiring further follow-up is a locus residing in AFF3 (2q11.2, P = 2.1 x 10-6), a gene previously implicated in childhood leukemia.
Conclusion
We have validated that NUDT15 rs116855232 is a locus with strong association with 6-MP tolerable dose, and it currently represents the most meaningful clinically predictive marker in Japanese. These findings indicated that NUDT15 genotyping should be considered prior to initiation of 6-MP treatment. Prospects for the identification of additional loci of 6-MP tolerance are high after further validation of suggestive loci observed in this Japanese study.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract