Background. Maintenance of induced responses remains a significant unmet medical need in patients with acute myeloid leukemia (AML) or high risk myelodysplastic syndrome (MDS) not eligible for allogeneic hematopoietic stem cell transplantation (HSCT). The allogeneic leukemia-derived dendritic cell vaccine, DCP-001, has shown to generate both humoral and cellular immune responses and is safe for clinical practice (van de Loosdrecht et al., Cancer Immunol. Immunother. 2018). In this study we show additional data on long-term follow up and patient-related risk factors from the phase 1 study.
Methods. AML- or MDS-patients (n=12), ineligible for HSCT, were included in a post-hoc clinical analysis of the DCP-001 phase I trial, receiving DCP-001 vaccination at the Amsterdam University Medical Center, VU University Medical Center, Amsterdam, the Netherlands (NCT01373515). All clinical data, including baseline characteristics as cytogenetics, pre-and post-vaccination treatment and morphologic blast counts in follow up, were retrospectively collected from corresponding patient files. Targeted next generation sequencing (NGS) gene panel data and cytogenetics were utilized to determine (cyto-)genetic risks following the European Leukemia Net (ELN) 2017 criteria. We defined 'response' as decreased or stable morphologic blast counts in bone marrow at day 126 (last day of official study evaluation) compared to study entry.
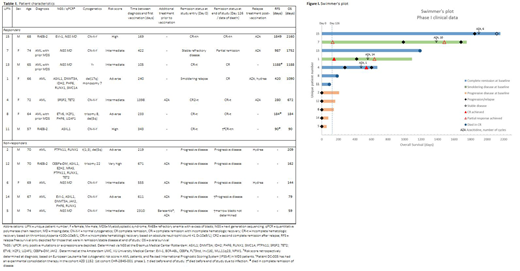
Results. Seven out of twelve patients responded to treatment, the other patients showed progressive disease (data are summarized in table 1). The median relapse free survival (RFS) for the responding patients was 420 days (range 90-1849) and overall survival (OS) was 1090 days (90-2160); two patients died early in complete remission (CR) due to infection (respectively at 90 and 184 days). Notably, three of these patients were intermediate risk and four were poor risk patients based on the ELN risk classification for AML or Revised International Prognostic Scoring System (IPSS-R) for MDS. The median OS for the non-responders was 144 days (range 59-209). These five patients had relapsed or refractory disease at start of vaccination with detectable circulating peripheral blasts; four of them received additional azacitidine (AZA) therapy prior to vaccination. Moreover, they were vaccinated after induction and consolidation therapy with a median time between diagnosis and first vaccination of 611 days (219-2310) compared to 240 days (105-1398) for the seven responding patients. Of the latter, all were in complete remission (CR) (n=5) or had morphologic marrow blast counts <10% (n=2) at the start of vaccination. Two responders that progressed and two that relapsed after vaccination were treated with AZA, resulting in one complete remission and two partial responses (one non-evaluable due to sample failure), see figure 1.
Conclusion/discussion. Clinical data of the phase 1 trial show that DCP-001 may prolong duration of remission or smoldering disease in intermediate and high risk AML or MDS patients. Response was associated with treatment by vaccination shortly after achieving CR. This should be taken into account in selection criteria of future vaccination studies. Hence, an international multi-center phase 2 study (ADVANCE II; NCT03697707) is currently being conducted to determine the effect of DCP-001 on measurable residual disease (MRD) in AML patients, aiming to convert them to a MRD negative state. Furthermore, we demonstrated that AZA can be applied as rescue therapy upon progression after vaccination.
Rovers:DCprime: Employment. Cloos:Daiicchi Sankyo: Speakers Bureau; Glycomimetics: Research Funding; Takeda: Research Funding; Novartis: Other: MRD assessments of clinical trials, Research Funding; Merus: Other: MRD assessment of a clinical trial , Research Funding; Genentech: Consultancy, Research Funding; Helsinn: Other: MRD assessment of a clinical trial; DCPrime: Other: MRD assessment of a clinical trial. de Gruijl:DCprime: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract