Background: The mechanisms of resistance to immunotherapies in patients with acute myeloid leukemia (AML) are not well characterized and biomarkers for improved immunotherapeutic strategies are critical. Multiple phase II/III clinical trials combining hypomethylating agents (HMA), azacitidine (AZA) and decitabine, with immune checkpoint inhibitors are now underway for patients with newly diagnosed and relapsed/refractory (R/R) AML and MDS based on data suggesting that 1) hypomethylating agents increase tumor expression of PD-L1 and PD-1 in myeloid malignancies(Yang H et al. Leukemia 2014); 2) blocking the PD-1/PD-L1 signaling axis has profound anti-leukemic response (Zhou et al. Blood 2011); and 3) clinical responses to Azacitidine/PD1 inhibitor (nivolumab) in relapsed AML (Daver et al., Cancer Discovery). Here, we characterize the baseline immune landscape and potential mechanisms of resistance using single-cell mass cytometry (CyTOF) profiling of serially collected samples from R/R AML patients undergoing therapy with AZA and PD-L1 inhibitor avelumab [NCT02953561].
Methods: Bone marrow (BM) and peripheral blood (PB) samples were collected from 9 patients prior to treatment with AZA and avelumab and after cycles 1, 3, and 5 (as available). To interrogate immune profiling in these samples, we have developed and optimized a novel CyTOF antibody panel that includes immunophenotypic markers to distinguish AML stem cells and blasts from adjacent immune cell subsets (T, B, NK cells, and monocytes), as well as known checkpoint ligands and receptors. Data was analyzed using the uniform manifold approximation and projection (UMAP) algorithm (McInnes et al. arXiv 2018) implemented through Cytofkit (Chen et al. PLoS Comput Biol 2016).
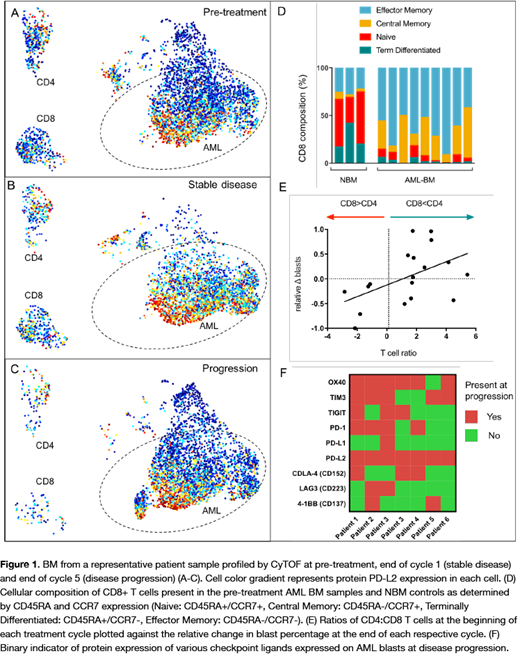
Results: We first used the multi-parameter immunophenotyping to characterize the immune microenvironment in normal and leukemic BM prior to therapy. At baseline, the composition of AML BM CD4 and CD8 T cells contained a significantly smaller fraction of naïve T-cells when compared to healthy bone marrow controls (both p<0.001). The proportion of terminally differentiated CD8+ cells was also significantly less in AML BM (p<0.001). Conversely, effector memory CD4 and CD8 cells comprised the major fraction of T-cells in AML BMs (Fig 1D). Due to the small number of patients we investigated response to therapy on a cycle-by-cycle basis rather than overall patients' outcomes. By this approach, we found that the relative ratio of T cells to blasts did not significantly predict response, however, patients with blast reduction or stable disease after 1 cycle of treatment had significantly lower CD4:CD8 ratios (i.e. more CD8 cells per CD4 cell) than those who progressed (p=0.04). In fact, CD4:CD8 ratio at the beginning of the cycle was significantly correlated with relative blast reduction in the bone marrow (R2=0.25, p=0.035) (Fig 1E). This is consistent with what has been seen in other cancer types where low CD4:CD8 has been associated with favorable prognosis (Sato et al. PNAS 2005). Baseline PD-L1 expression levels in these patient samples was low and did not predict response to therapy. While 4 of the 9 patients experienced and initial blast reduction, 7 developed disease progression while on the trial. Serial measurements from the same patients allowed us to track both resistant and newly emerging clones over the course of therapy (representative patient; Fig 1A-C). While PD-L1 levels were consistently low, we did observe high PD-L2 protein expression in AML cells resistant (the 7 with progressive disease) to HMA/PD-L1 inhibition, and PD-L2 was also frequently expressed in the emerging clones not present at baseline. We profiled the AML clones from each patient present at disease progression for the expression of other known checkpoint ligands and receptors (Fig 1F). Expression of PD-L2, OX40, and TIM-3 was detected in the majority of these resistant clones (100%, 86%, and 71%, respectively).
Conclusions: Single-cell characterization of R/R AML reveals that the immune landscape of these patients at baseline is significantly altered when compared to healthy bone marrow. The ratio of CD:/CD8 and composition of residual T cells appear to be the most important predictors of response to HMA/PD-L1 inhibition. Finally, AML cells express a variety of other immune checkpoints, particularly PD-L2 but also OX40 and TIM3, that should be considered for future combination therapy.
Short:AstraZeneca: Consultancy; Amgen: Honoraria; Takeda Oncology: Consultancy, Research Funding. Konopleva:Astra Zeneca: Research Funding; Reata Pharmaceuticals: Equity Ownership, Patents & Royalties; Ablynx: Research Funding; Ascentage: Research Funding; Kisoji: Consultancy, Honoraria; Genentech: Honoraria, Research Funding; Amgen: Consultancy, Honoraria; F. Hoffman La-Roche: Consultancy, Honoraria, Research Funding; Cellectis: Research Funding; Eli Lilly: Research Funding; AbbVie: Consultancy, Honoraria, Research Funding; Stemline Therapeutics: Consultancy, Honoraria, Research Funding; Forty-Seven: Consultancy, Honoraria; Calithera: Research Funding; Agios: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.