Introduction:
EPOCH-based chemotherapy is considered a preferred first-line treatment for HIV-associated diffuse large B-cell lymphoma (DLBCL) and primary effusion lymphoma in the 2019 NCCN guidelines. We previously reported that adding the HDAC inhibitor vorinostat (VOR) to EPOCH had no impact on complete response (CR) rate, the primary trial endpoint, and similar 1-year survival rates (ASCO 2018, abstract 7573). Here, we report updated results from AMC075 and evaluate the impact of a DTI ≥15 days on clinical outcomes. A DTI ≥15 days has been shown to be associated with a better prognosis in an HIV-negative population treated with R-CHOP (Maurer et al. J Clin Oncol 2018; 36:1603-1610). We also look at the impact of allowing 1 cycle of systemic therapy given prior to study enrollment in order to circumvent logistical challenges in recruiting otherwise eligible trial subjects.
Methods:
Between 2012 and 2017 we conducted a randomized phase II study of EPOCH (with rituximab in CD20+ tumors) ± VOR 300 mg administered on days 1-5 of each cycle with HIV antiretroviral therapy in 90 participants with aggressive HIV-NHLs. Eligible patients had at least one of the following high-risk features or tumor characteristics: Age-adjusted International Prognostic Index (aa-IPI) of 2-3, Ki-67 ≥ 80%; activated B-cell (ABC) diffuse large B-cell lymphoma(DLBCL); or any other aggressive non-germinal center (non-GCB), non-Burkitt B-cell NHL. Patients who received 1 prior cycle of chemotherapy (CHOP-like or EPOCH) +/- rituximab were allowed to register. The primary endpoint was CR rate. Secondary objectives included determining adverse events, survival rates, and the effect of EPOCH +/- VOR on HIV viral reservoirs. We performed a post-hoc analysis evaluating the impact of time from DTI ≥15 days vs. <15 days on clinical outcomes and that of pre-protocol systemic therapy.
Results:
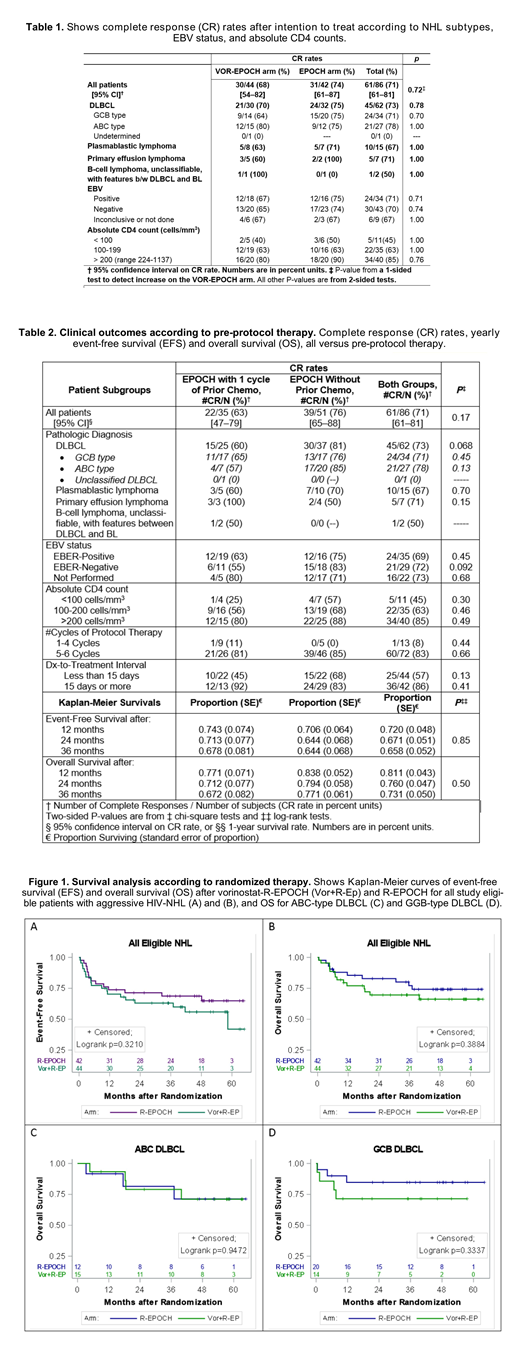
Of 86 evaluable patients, 64 had DLBCL (34 GCB-type and 27 ABC-type), 15 plasmablastic lymphoma (PBL), and 7 primary effusion lymphoma (PEL) [Table 1]. Fifty-one (59%) patients were treatment naïve, whereas 35 (41%) had been pre-treated with one cycle of CHOP-like or EPOCH prior to enrollment. Grade 4 neutropenia was more frequent for VOR-EPOCH than for EPOCH (47% vs. 20%), and also grade 4 thrombocytopenia more frequent for VOR-EPOCH (29% vs 2%). The two regimens had similar rates of febrile neutropenia (18% vs. 16%). CR rates were also comparable for the two regimens (68% vs. 74%) [1-sided P=0.72] (Table 1). Median HIV plasma RNA generally decreased in both arms. No significant differences were observed in T-cell subsets or size of the latent HIV reservoir between arms. Treatment outcomes did not differ significantly across NHL subtypes or EBV status (Table 1), nor with pre-protocol systemic therapy (Table 2). Overall CR rates were 45% in patients with CD4+ counts of 50-100 cells/mm3, 63% with 100-199 cells/mm3, and 85% in patients with >200 cells/mm3(Table 1). The 3-year overall survival rates for VOR-EPOCH was 70% and for EPOCH 77% (log-rank P=0.39), and 3-year event-free survival rates were 63% vs. 69%, respectively (log-rank P=0.32) [Figure 1]. Thirty-six patients (86% of n=42) with long DTI (≥15 days) had CR, compared to only 25 (57% of n=44) with short (<15 days) DTI (P=0.0032). The proportion of patients with short DTI was somewhat higher (63%) in the pre-treated group than in the treatment-naïve group (43%; P=0.072) without significant difference in CR or survival rates (Table 2).
Conclusion:
This prospective trial demonstrated that EPOCH-based chemotherapy was broadly efficacious against aggressive HIV-NHLs, including high risk subtypes (ABC DLBCL, PBL, and PEL). However, a 5-day course of VOR given with each chemotherapy cycle failed to improve outcomes. Consistent with prior reports in HIV-uninfected patients with DLBCL, a DTI of ≥15 days was associated with better clinical outcomes than a shorter DTI. A strategy of permitting pre-protocol therapy was useful in facilitating accrual and was used in about 40% of study participants without compromising clinical outcomes.
Baiocchi:Prelude: Consultancy. Noy:Janssen: Consultancy; Medscape: Honoraria; Prime Oncology: Honoraria; NIH: Research Funding; Pharamcyclics: Research Funding; Raphael Pharma: Research Funding.
Vorinostat is being tested in combination with chemotherapy for HIV-associated B-cell lymphoma
Author notes
Asterisk with author names denotes non-ASH members.