BACKGROUND:
Resistance to cell death and metabolic reprogramming are common features of tumor cells. Although the introduction of selective BCR/ABL tyrosine kinase inhibitors (TKIs) has dramatically improved the outcomes and survival rates of chronic myeloid leukemia (CML) patients, some patients (20-30%) develop TKI resistance. The most aggressive and treatment-resistant CML is the subtype harboring BCR/ABL with the T315I mutation, and this subtype is refractory to nearly all TKI-induced apoptosis. Thus, alternative approaches that induce apoptosis-independent cell death are thought to compensate for apoptotic-resistant cells. Recently, necroptosis (also called programmed necrosis), which is generally driven by RIPK1/RIPK3/MLKL activation, has been demonstrated to be a new type of programmed cell death mode that is different from apoptosis. Thus, a deeper understanding of the molecular mechanisms regulating necroptosis might lead to the development of new therapeutic strategies that could remarkably improve the treatment-responses and outcomes of patients with TKI-resistant CML.
RESULTS:
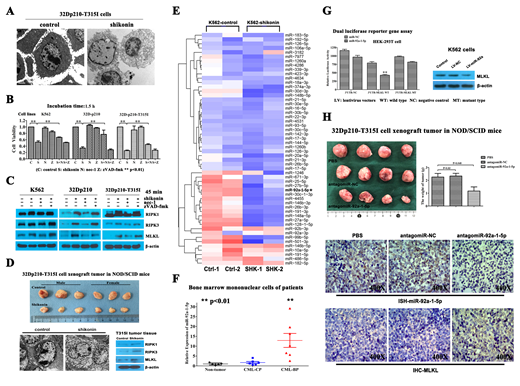
Shikonin, a compound purified from traditional Chinese medicine, has been reported to induce cell death in various tumor cell lines via a wide range of mechanisms. In our current study, we found that shikonin can effectively inhibit proliferation and induce necrosis-like morphological alterations (Fig. A and B) accompanied by RIPK1/RIPK3/MLKL signaling activation ((Fig. C) in CML cell lines, including the T315I mutant type (32Dp210-T315I). The effects of shikonin can be attenuated by the necroptosis-specific inhibitor (essentially a RIPK1 inhibitor) Nec-1, but not by the pan-apoptosis inhibitor z-VAD-fmk, indicating the occurrence of necroptosis in these cells ((Fig. B and C). Our data also show that shikonin has in vivo anti-CML activity via necroptosis induction in 32Dp210-T315I cells xenografted into NOD/SCID mice via subcutaneous injection ((Fig. D).
miRNAs play an important role in tumorigenesis mainly via regulation of gene expression. Our next generation sequencing-based microRNA expression profiling showed significant dysregulation of miR-92a-1-5p expression in a shikonin-treated CML cell line (K562) (Fig. E). We then measured the miR-92a-1-5p expression levels in bone marrow samples from CML patients and patients with nonhematologic malignant diseases. The data showed that the miR-92a-1-5p expression level was higher in primary cells obtained from CML-BC patients than in those from non-CML-CP patients, suggesting that miR-92a-1-5p upregulation is correlated with poor outcomes (Fig. F). Bioinformatics analyses and a dual luciferase reporter gene assay proved that MLKL, a downstream factor in the necroptosis pathway that usually acts as the necroptosis executor, is a direct target of miR-92a-1-5p (Fig. G). Overexpression of miR-92a-1-5p in vitro led to decreased MLKL protein abundance in CML cells (Fig. G). Inhibition of miR-92a-1-5p via use of a specific antago-miRNA could inhibit CML xenograft tumor growth and induce necroptosis via MLKL upregulation in vivo (Fig. H). Hence, we believe that miR-92a-1-5p plays a role in promoting the proliferation and survival of CML via downregulating the abundance of MLKL, the necroptosis executor.
CONCLUSIONS:
In conclusion, our study proves that shikonin can overcome TKI resistance and induce necroptosis in CML cells, mainly via a mechanism involving RIPK1/RIPK3/MLKL activation. Our study also suggests that miR-92a-1-5p is frequently overexpressed in CML patients with poor outcomes and that it can promote tumor survival by inhibiting MLKL expression. For the first time, we demonstrated that miR-92a-1-5p inhibition via antago-miRNA can potentially be used to treat CML via necroptosis induction. Since necroptosis has not yet been considered to be a therapeutic strategy for tumor treatment, our research confirms that it might indeed serve as a new modality to better control drug-resistant CML.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.