Background:
Richter Transformation (RT) is defined as the transformation of chronic lymphocytic leukemia (CLL) or small lymphocytic lymphoma (SLL) into an aggressive lymphoma. Diffuse large B-cell lymphoma (DLBCL) makes up the vast majority of RT and these patients have a poor prognosis compared to de novo DLBCL (Rossi D, et al. Blood, 2011; 117(12):3391-3401). Prognostic factors include therapy for CLL/SLL prior to transformation, TP53/CDKN2A abnormalities, as well as clonal relationship between CLL/SLL and RT. There are no randomized controlled trials to direct treatment of RT and many centers use clonal relationship to direct treatment decisions including whether or not patients should receive consolidation with autologous or allogeneic stem cell transplantation (Parikh SA, et al. Blood; 123(11): 1647-1657). One method for establishing clonality involves genetic analysis of the immunoglobulin heavy variable chain (IgHV) gene in both the CLL/SLL portion as well as the RT portion by means of IGHV V-D-J sequencing.
Whole genome Chromosomal microarray (CMA) provides a method of tracking duplications or deletions of chromosomal segments sometimes referred to as copy number variants (CNVs). CMA is used at our institution to establish chromosomal aberrations at diagnosis for all CLL/SLL patients. This provides prognostic information at diagnosis as well as the ability to track aberrations in the event of progression or transformation of disease. Use of CMA at diagnosis of CLL/SLL and upon transformation to DLBCL (RT) provides a method to determine clonal relationship. It also identifies aberrations involving TP53 and CDKN2A, which are known to be markers of poor prognosis.
Methods:
We performed a single center retrospective analysis of all patients at MUSC with pathologically confirmed CLL or SLL and Richter transformation between 1/01/2010 to 02/14/2019. We collected baseline demographic, clinical, laboratory, pathology, and outcomes data from the electronic medical record. Chromosomal Microarray Analysis (CMA) was performed on genomic DNA extracted from peripheral blood and fresh or formalin fixed paraffin-embedded lymph node samples using the Infinium HD Human Omni1 BeadChip or the CytoSNP-850K v1.1 BeadChip Array (Illumina, Inc., San Diego, CA). Copy number and genotype data were analyzed using NxClinical (BioDiscovery, Inc) software. Aberrations that were in 100% of cells were considered constitutional and were not included in the data analysis; whereas regions noted to be in less than 100% of cells were considered clonal changes.
Results:
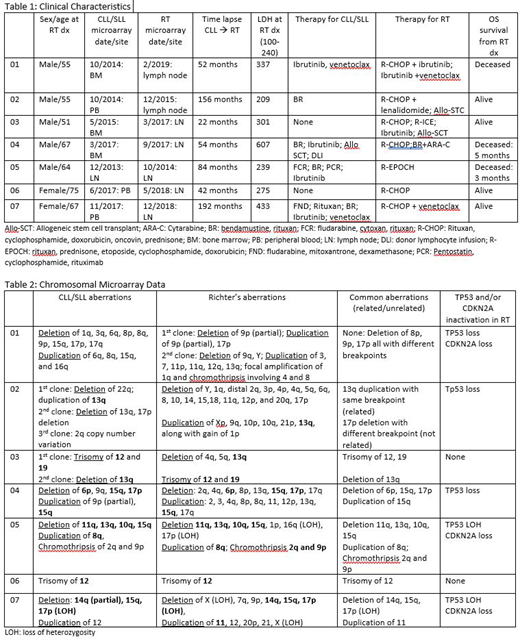
We identified a total of 24 patients with a diagnosis of Richter transformation to DLBCL with prior history of CLL/SLL. Of these patients, 7 had CMA performed on both the CLL/SLL and RT samples. Clinical characteristics were collected for each patient and are included in table 1. CMA data including deletions, duplications, LOH, loss of TP53 and/or CDKN2A is displayed for each patient in table 2. Six of the 7 patients (85%) had common CMA aberrations identified in the CLL/SLL and RT samples providing evidence of a common clonality. Five of the 7 patients (71%) were identified to have TP53 loss and/or CDKN2A loss by CMA at the time of RT diagnosis.
Discussion:
Chromosomal microarray was able to provide proof of common clonality in 6 of 7 patients with Richter transformation to DLBCL, which is a poor prognostic feature of RT. It also identified a loss of TP53 and/or CDKN2A in 5 of 7 patients which is also a poor prognostic feature of RT. This information can be used to counsel patients on prognosis and could effect clinical recommendations such as treatment with Allogeneic stem cell transplantation. Institutions with the ability to run CMA should utilize this modality in patients Richter transformation to DLBCL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.