Introduction: About 20-40% of patients (pts) with multiple myeloma (MM) present with moderate or severe renal impairment (RI) and about 25% of pts will also experience RI later during the disease course (Dimopoulos MA, et al; Leukemia 2008; 22:1485-1493). Moderate or severe RI is associated with poorer overall survival (OS) and higher risk of early death but also with challenges in the management and administration of the appropriate treatment. Daratumumab, an IgG1κ human monoclonal antibody that targets CD38, has shown efficacy and a favorable safety profile in pts with relapsed or refractory MM (RRMM) both as a monotherapy (Lonial S, et al; Lancet 2016; 387(10027):1551-1560) and in combination with other anti-myeloma agents (Dimopoulos MA, et al; N Engl J Med 2016; 375:1319-1331, and Palumbo A, et al; N Engl J Med 2016; 375:754-766). In population pharmacokinetic analyses, no clinically important differences in exposure to daratumumab were observed between pts with renal impairment and those with normal renal function. However, there are no prospective data on the safety and efficacy of daratumumab in pts with RRMM and severe renal dysfunction or those requiring dialysis.
Methods: DARE is an ongoing multicenter, single arm, open-label, phase 2 study, aiming to enroll ~38 adult pts with documented RRMM and severe renal impairment (estimated glomerular filtration rate [eGFR]< 30 ml/min/1.73m2) or in need for hemodialysis. Pts must previously have had ≥ 2 lines of therapy with both bortezomib- and lenalidomide-based regimens and an Eastern Cooperative Oncology Group performance status (ECOG PS) score of ≤ 2. Exclusion criteria include previous treatment with daratumumab or other anti-CD38 therapy. All pts receive 28-day cycles of treatment with daratumumab given intravenously at 16 mg/kg weekly for Cycles 1-2, every 2 weeks for Cycles 3-6, followed by every 4 weeks thereafter, with dexamethasone 40 mg given weekly for each 4-week cycle. The primary endpoint of the study is the evaluation of progression-free survival (PFS). Key secondary endpoints include overall response rate (ORR; defined as the proportion of pts with partial response or better), renal response rate (RRR; defined as the proportion of pts with best response of renal partial response or better), and the assessment of daratumumab safety and tolerability. All responses were based on investigators' assessment per International Myeloma Working Group criteria. This interim analysis presents study results for pts who received the first dose of study treatment at least 3 months prior to the cut-off date (06/05/2019).
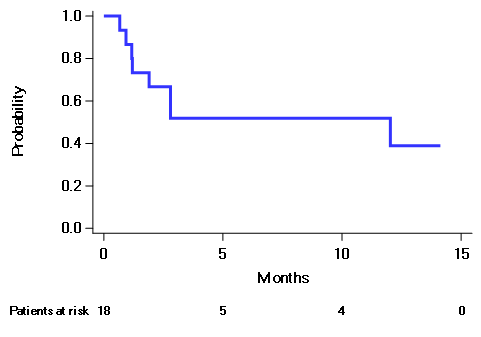
Results: Eighteen pts, enrolled in 4 centers, were included in this prespecified analysis. The pts' median age was 74.0 years, and most were males (77.8%). The median time from MM diagnosis to first dose of daratumumab was 3.6 years. The number of pts with baseline ECOG PS 0, 1, and 2 were 4 (22.2%), 13 (72.2%), and 1 (5.6%), respectively. At the start of the study, 22.2% and 77.8% of patients had ISS Stage II and III disease, respectively. Moreover, 44.4%, and 55.6% of pts had a revised ISS stage II and III, respectively. Median number of prior lines of therapy was 3.5, and two (11.1%) pts had previous autologous stem cell transplantation; Median eGFR at baseline was 12 mL/min/1.73m2. The median number of therapy cycles received per patient was 4.5. The median time from first daratumumab dose to first partial response or better was 0.9 months. The median follow-up is 4.4 months and the Kaplan-Meier estimate of the 6-month PFS rate is 51.9% (Figure), ORR was 44.4% (8/18 pts) (including VGPR in 4, and PR in 4 pts), and RRR was 27.8% (5/18 pts). By the cut-off date, 10 (55.6%) pts were still on daratumumab; 7 (38.9%) pts discontinued treatment due to progressive disease and 1 (5.6%) due to a fatal serious adverse event (SAE). Overall, ten (55.6%) pts had ≥ 1 AE of grade 3 or 4. Of all grade 3 or 4 AEs, the most frequent were anemia (4, 25%), hyperglycemia (3, 18.8%), and hypocalcemia (2, 12.5%). Three (16.7%) pts suffered a single SAE each: lung infection, sepsis (fatal), and stroke.
Conclusions: The combination of daratumumab with dexamethasone is efficacious and with a favorable safety profile and no new safety signals, in pts with RRMM with severe renal impairment. Importantly, hematologic responses are high in these heavily pretreated patients while about 28% achieved also a renal response. The study is ongoing and updated results will be presented at the meeting.
Kastritis:Pfizer: Honoraria; Prothena: Honoraria; Genesis: Honoraria; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Research Funding; Takeda: Honoraria. Terpos:Takeda: Honoraria, Other: Travel expenses, Research Funding; Genesis: Honoraria, Other: Travel expenses, Research Funding; Celgene: Honoraria; Medison: Honoraria; Amgen: Honoraria, Research Funding; Janssen: Honoraria, Other: Travel expenses, Research Funding. Symeonidis:Novartis: Membership on an entity's Board of Directors or advisory committees, Research Funding; Gilead: Membership on an entity's Board of Directors or advisory committees, Research Funding; Janssen: Membership on an entity's Board of Directors or advisory committees, Research Funding; Pfizer: Research Funding; Roche: Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi: Research Funding; Tekeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; MSD: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding. Delimpasi:Genesis: Honoraria, Other: Travel grant; Janssen: Honoraria; Takeda: Honoraria; Amgen: Honoraria. Cavo:Janssen, Celgene: Other: Travel Accommodations; Janssen, Celgene, Amgen, Abbvie: Consultancy, Membership on an entity's Board of Directors or advisory committees; Janssen, Celgene: Speakers Bureau; Celgene, Janssen, Amgen, BMS, Abbvie, Takeda: Honoraria. Zamagni:Celgene Corporation: Honoraria, Other: Advisory board, Speakers Bureau; Janssen: Honoraria, Other: Advisory board, Speakers Bureau; Amgen: Honoraria, Other: Advisory board, Speakers Bureau; BMS: Honoraria, Other: Advisory Board, Speakers Bureau; Takeda: Honoraria, Speakers Bureau; Sanofi: Honoraria, Other: Advisory Board, Speakers Bureau. Katodritou:Takeda: Honoraria; Janssen: Honoraria; Genesis: Honoraria; Amgen: Honoraria. Gamberi:Janssen: Honoraria, Membership on an entity's Board of Directors or advisory committees; Celgene: Honoraria, Membership on an entity's Board of Directors or advisory committees; BMS: Honoraria, Membership on an entity's Board of Directors or advisory committees; Amgen: Honoraria, Membership on an entity's Board of Directors or advisory committees. Gavriatopoulou:Genesis: Honoraria, Other: Travel expenses; Amgen: Honoraria; Janssen: Honoraria, Other: Travel expenses; Takeda: Honoraria, Other: Travel expenses. Hatjiharissi:Janssen: Honoraria. Dimopoulos:Sanofi Oncology: Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract