Background: The current standard of care in MM, lenalidomide (LEN), is frequently used as part of first-line (1L) treatment (tx) or as maintenance tx following autologous stem cell transplantation; therefore, there is a growing need for appropriate tx options in patients (pts) with relapsed/refractory MM (RRMM) previously treated with LEN. OPTIMISMM is the first and only phase 3 trial in early RRMM (median 1-2 prior lines) to specify prior LEN tx as an inclusion criterion. The trial showed a significant improvement in progression-free survival (PFS) with pomalidomide + bortezomib (BORT) + dexamethasone (DEX) (PVd) vs BORT + DEX (Vd) (median PFS 11.2 months (mo) [95% confidence interval (CI): 9.66-13.73] vs 7.1 mo [5.88-8.48]; hazard ratio [HR] = 0.61, 95% CI: 0.49-0.77; P < 0.0001). How the PVd results from the OPTIMISMM trial compare with results achieved with other tx options for LEN-exposed pts with RRMM has not been established.
Aim: This analysis aimed to put the phase 3 OPTIMISMM (PVd) trial results into perspective by comparing with results achieved for other tx options post LEN.
Methods: A systematic literature review (SLR) was conducted in May 2018 and updated in Dec 2018, in line with National Institute for Health and Care Excellence (NICE) and Cochrane guidelines, to identify randomized controlled trial (RCT) data on efficacy outcomes in LEN-exposed pts with early RRMM. Electronic database searches were performed in Embase®, MEDLINE, and the Cochrane Library, and study eligibility criteria were defined using the PICOS framework. Searches were restricted to Jan 2004 onward.
Descriptive statistics were used to assess between-trial heterogeneity in study design, baseline demographics, and clinical characteristics. Where the evidence network and heterogeneity assessment suggested an indirect treatment comparison (ITC) was feasible, the analysis was conducted in the Bayesian framework, according to the NICE Decision Support Unit guidelines.
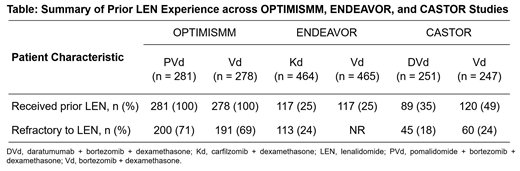
Results: ENDEAVOR and CASTOR were the only relevant trials that reported PFS in pts with RRMM previously treated with LEN. Comparator txs included carfilzomib + DEX (Kd) (ENDEAVOR) and daratumumab + BORT + DEX (DVd) (CASTOR). OPTIMISMM was designed to prospectively evaluate pts who had prior LEN, whereas ENDEAVOR and CASTOR reported only pt subgroups with prior LEN; all studies reported the number of pts who were refractory to LEN, with values varying from 18% (DVd) in CASTOR to 71% (PVd) in OPTIMISMM (Table).
Differences in prognostic baseline characteristics were noted between the overall study populations in OPTIMISMM and ENDEAVOR; as the corresponding data were not available for CASTOR, a full assessment of heterogeneity was not possible.
Although Vd initially seemed to link the network of evidence, the comparator arm of the CASTOR trial had a fixed 8-cycle Vd tx duration, whereas pts randomized to the comparator arm in OPTIMISMM and ENDEAVOR received Vd continuously until disease progression. As the Vd arms could not be considered comparable, DVd was excluded from the ITC.
Based on data from ENDEAVOR and OPTIMISMM, PVd could only be compared with Kd and Vd. Based on the ITC, Vd was associated with a statistically significant shorter PFS vs PVd (HR PVd vs Vd = 0.62, 95% credible interval [Crl]: 0.50-0.76) in the prior-LEN RRMM population. No statistically significant difference was observed in PFS for Kd vs PVd (HR PVd vs Kd = 0.90, 95% Crl: 0.62-1.28). It is important to note that pt characteristics vary between these trials, particularly regarding prior LEN.
Discussion: Due to increasing use of LEN in the 1L setting and as maintenance tx, a growing population of pts with early RRMM are treated with LEN. This SLR found that OPTIMISMM is the only study to date to prospectively investigate the efficacy of regimens in this population. Only 2 other RCTs were identified that reported data for pts with prior LEN, and in both cases, these were subgroups of the overall trial population, thus limiting the robustness of the comparator data. HRs for PFS from the ITC aligned with those from OPTIMISMM, confirming the superiority of PVd over Vd. The ITC between PVd and Kd found no statistically significant difference between these regimens. Comparison with DVd was not possible given the differences in design between CASTOR and OPTIMISMM. Further studies in pts previously treated with LEN are warranted, given the impact of prior tx on outcomes for pts with early RRMM.
Weisel:Janssen: Consultancy, Honoraria, Research Funding; GSK: Honoraria; Takeda: Consultancy, Honoraria; Sanofi: Consultancy, Honoraria, Research Funding; Adaptive Biotech: Consultancy, Honoraria; Celgene Corporation: Consultancy, Honoraria, Research Funding; Bristol-Myers Squibb: Consultancy, Honoraria; Amgen: Consultancy, Honoraria, Research Funding; Juno: Consultancy. Dhanasiri:Celgene Corporation: Employment, Equity Ownership. Gauthier:Amaris Consulting: Employment, Equity Ownership; Celgene Corporation: Consultancy. Padhiar:Amaris Consulting: Employment. Casal:Celgene Corporation: Employment. Richardson:Oncopeptides: Membership on an entity's Board of Directors or advisory committees, Research Funding; Celgene: Membership on an entity's Board of Directors or advisory committees, Research Funding; Takeda: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bristol-Myers Squibb: Research Funding; Karyopharm: Membership on an entity's Board of Directors or advisory committees; Amgen: Membership on an entity's Board of Directors or advisory committees; Sanofi: Membership on an entity's Board of Directors or advisory committees; Janssen: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.