Introduction: Iron Refractory Iron Deficiency Anemia (IRIDA) is an autosomal recessive iron metabolism disorder caused by mutations in Tmprss6 gene which encodes for Matriptase2 (MT2) that, by activating hemojuvelin (HJV), regulates the production of hepcidin, the master iron regulatory hormone. Altered MT2 cannot suppress hepatic BMP6/SMAD signaling in low iron condition, hence the resulting hepcidin excess blocks dietary iron absorption and cells release, leading to a form of iron deficiency that is typically refractory to oral iron supplementation. IRIDA is characterized by moderate/severe microcytic anemia (Hemoglobin 6-9 g/dL; MCV 45-65 fL); low transferrin saturation (<5%); impaired oral iron absorption and only a transient response to parenteral iron. Nonetheless, the current treatment is mainly based on parenteral iron therapy. A case study on a child with IRIDA showed for the first time the ability of Sucrosomial® Iron, to increase hemoglobin and MCV values over time (Capra et al., 2017). This oral iron formulation is an innovative preparation of ferric pyrophosphate, covered by a phospholipids plus sucrester matrix, with gastro-resistance properties, high bioavailability and tolerability due to alternative absorption pathways as endocytosis and M cells mediated route (Gomez-Ramirez et al., 2018). Moreover, Sucrosomial® Iron has been successfully used to treat iron deficiency in various clinical conditions, including inflammatory bowel diseases (Abbati et al., 2019). To confirm and characterize the ability of Sucrosomial® Iron to increase Hb in IRIDA disease we studied the response to Sucrosomial® Iron in a IRIDA mouse model (Mask) comparing the efficacy of Sucrosomial® Iron and Sulfate Iron at two different doses and in chronic treatment.
Aim: to study Sucrosomial® Iron effect in IRIDA using the Tmprss6 knock-out mouse model
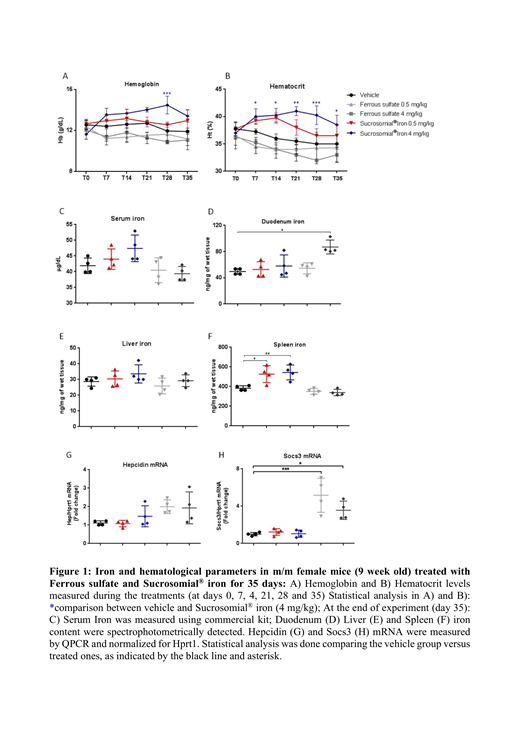
Material and Methods: m/m homozygous mice (9-weeks old male mice, four mice per experimental group) were kept at iron balance diet and treated with 0.5 or 4 mg/Kg of Ferrous sulfate, Sucrosomial® Iron (patent n° PCT/IB2013/001659 owned by Alesco s.r.l, Italy), or vehicle by gavage for 35 days. Four 9-weeks old m/- male mice per experimental group were daily treated and Hb and Ht were monitored weekly. Mice were sacrificed at the end of treatments; blood, and different organs were collected for analysis. Total RNA was isolated from tissues using TRIzol Reagent (Ambion), cDNA was generated by Reverse transcription (Promega, Milan, Italy) and samples were analyzed for Hepcidin and Socs3 mRNA levels by qRT-PCR using PowerUp SYBR Green Master Mix (Life Technologies).
Results: we analyzed the iron status of anemic homozygous Mask mice from 3 to 35 weeks of age by studying serological and tissue iron content. Interestingly only Sucrosomial® Iron (not Ferrous Sulfate), increased hemoglobin level from 11-12 to 13-14 g/dL in the first week with a tendency to increase until the fourth week, when it stabilized at 13 g/dL (Figure 1A-B). Serum iron concentration was higher in the Sucrosomial® Iron treated animals than in those treated with vehicle, while was lower in the Ferrous sulfate treated animals. Similar pattern was observed for spleen iron content that increased in mice treated with Sucrosomial® Iron but not in those receiving Ferrous sulfate. Liver iron concentration did not apparently varied after the treatments, but duodenal iron increased significantly only in the mice treated with the higher dose of Ferrous sulfate (Figure 1 C-F). Interestingly, we found that the mice treated with both doses of Ferrous sulfate, but not those treated with Sucrosomial® Iron, had a higher mRNA levels of hepcidin and of the inflammatory marker Socs3 (Figure 1 G-H).
Conclusion: this study showed for the first time that Sucrosomial® Iron is able to increase hemoglobin level in a mouse model of IRIDA, probably due to its alternative absorption pathway. Sucrosomial® Iron could be used as effective iron supplement to improve iron status in IRIDA patients.
Girelli:La Jolla Pharmaceuticals: Membership on an entity's Board of Directors or advisory committees; Novartis: Consultancy; Vifor Pharma: Other: honoraria for lectures; Silence Therapeutics: Membership on an entity's Board of Directors or advisory committees.
Author notes
Asterisk with author names denotes non-ASH members.