Several studies indicate that abnormal megakaryocytes (MK) drive bone marrow fibrosis in primary myelofibrosis (PMF). Since the lung is a site where MK lodge to release platelets (Lefrançais, Nature 2017; 544:105), we investigated whether the MK abnormalities observed in PMF also lead to fibrosis in lung.
To this aim, we used Gata1low mice that express MK abnormalities similar to those observed in PMF patients and develop myelofibrosis with age (Zingariello, Blood 2013;121:3345) and compared number/morphology of MK and extent of fibrosis in lungs from Gata1low mice or from their wild-type (WT) littermates. Two age groups were considered: adult, A (5-7 months) and old, O (>12 months). Pulmonary fibrosis was assessed by H&E and Masson's staining and graded from 0 (absent) to 3 (severe). MK were evaluated by immunohistochemistry and immunofluorescent analysis with CD42 and their number expressed per 0.144 mm2. Statistical analysis was performed with Shapiro test for normality while chi-square test and Spearman's correlation test for comparisons.
Since the mutation is carried in the CD1 background that is known to develop multisite inflammation with age (Brayton, Vet Pathol 2012; 49:85), preliminary experiments compared the pro-inflammatory signature of the mice groups by ELISA determinations of the plasma levels of CXCL1 (murine equivalent of IL-8) and its inducer lipocalin2 (LCN2). WT mice expressed a pro-inflammatory signature with high levels of LCN2 (90±45ng/mL in A; 50±49 in O) and CXCL1 (44.5±11.4pg/mL in A;31.6±4.6 in O compared to 17.0 ±5.1 in O P-Selnull/Gata1low mice which do not express inflammation, p<0.01). These levels were not affected by age. Gata1low mice expressed high levels of LCN2 that increased with age (100±18 in A and 150±45 in O, p<0.05) and levels of CXCL1 significantly lower than those expressed by WT (27.9±1.3 in A and 28.8 ±9.7 in O, p<0.05) but still greater than those in negative controls.
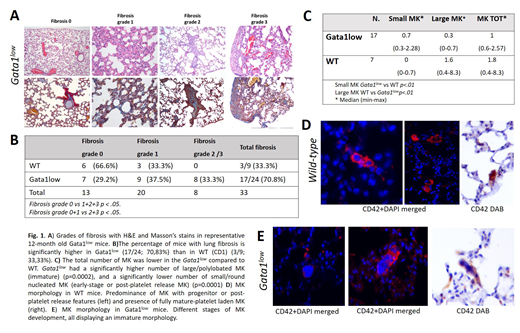
Mild and localized interstitial pneumonia characterized by infiltration with lymphocytes, plasma cells and macrophages in the alveolar septae and peribronchial-vascular spaces was observed in 66.6% of WT mice. However, mild interstitial pneumonia was detected in 84.6% of Gata1low mice where it was multi-focal to coalescent and extended from alveolar septae to subpleural interstitium. Lungs from 33% of WT mice had mild fibrosis with accumulation of collagen in alveolar septae (grade 1) while those from 70.8% Gata1low mice presented severe fibrosis (37.5% grade 1, 29.4% grade 2 and 17.6% grade 3) (Fig. 1A, 1B). The mild interstitial pneumonia associated to grade 1 fibrosis present both in Gata1low and WT littermates is considered a background lesion due to the pro-inflammatory cytokine profile of the strain investigated. By contrast, despite the severity of pneumonia was similar in Gata1low and WT littermates, grade 2 and 3 fibrosis was only present in Gata1low mice, indicating that it was not due to the baseline inflammatory milieu of their CD1 background.
MK were observed intravascularly and within the perivascular interstitium of lungs from both WT and Gata1low mice, the same location where fibrosis was detected. The number of MK was significantly lower in Gata1low than in WT littermates (median=1, range 0.6-2.57 vs 1.8, range 0.4-8.3, respectively, p=0.02) (Fig 1C). The morphology of MK found in the two groups was significantly dissimilar (p<0.01): most MK (88.8%) in WT lungs contained either a round nucleus with small amount of cytoplasm or a polylobated nucleus with well developed platelet territories (Fig.1D). The morphology of most (70%) of MK in Gata1low lungs was instead characterized by large size, polylobated nuclei but poorly developed platelet territories (Fig.1E). Therefore, the significantly higher number of Gata1low mice that exhibited moderate to severe pulmonary fibrosis compared to WT controls was associated with abnormal MK suggesting that factors released by Gata1low MK in addition to CXCL1/LPN2 are involved in establishing lung fibrosis in this model. Further studies will clarify whether these factors are similar to those responsible for fibrosis in bone marrow.
In conclusion, the significantly higher number of Gata1low mice that exhibited moderate to severe pulmonary fibrosis compared to WT controls establishes Gata1low mice as a potential new animal model to study human idiopathic pulmonary fibrosis and to identify possible drug targets to treat this disease.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.