Introduction:
Administration of increasing doses of FIX to hemophilia B (HB) mice reveals that the extravascular compartment is capable of binding several-fold more FIX than circulates in the plasma (Cooley et al., Blood 2019). FIX binds rapidly and reversibly to vascular endothelium and the extravascular matrix, in part mediated by interaction of the Gla domain with collagen IV (Col IV). Anticoagulant heparan sulphate and Col IV localize predominantly to the basement membrane and supramolecular structure analysis suggests that heparan sulphate chains are integrated into Col IV-containing networks. Previous work has demonstrated the contribution of the heparin and antithrombin (AT) binding exosites on the protease domain to the regulation of FIXa activity. We now compare the contribution of these protease exosites to in vivo recovery and clearance of the FIX(a) protease and zymogen in hemophilia A (HA) or HB mice.
Methods:
Recombinant human FIX variants expressed in HEK293 cells with substitutions in the AT- (R150A) and heparin-binding (K126A/K132A) exosites (chymotrypsinogen #) were purified to homogeneity and activated to FIXa with FXIa. FIX(a) pharmacokinetics (PK) in HA or HB mice were determined with injection of a weight-based dose into the tail vein. Serial retro-orbital blood samples were collected, plasma isolated and FIX(a) content determined with a human FIX specific ELISA. FIX(a) concentrations were plotted versus time and fit to the equation: [IX]t = [IX]max*(C1*e(-k1*t)+ C2*e(-k2*t)), where k1and k2are rate constants for the initial and terminal elimination phases, respectively. As the 4-parameter fit resulted in some large error estimates, the two phases were fit separately to estimate the respective parameters.
Results:
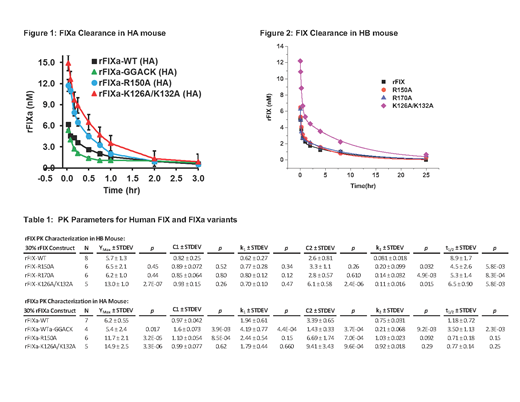
PK for clearance of predicted 30% plasma levels of human FIXa (5 min to 3 hr) or FIX (5 min to 25 hr) were examined in HA (Fig 1) or HB mice (Fig 2), respectively. HA mice were employed for FIXa to avoid in vivoclot formation triggered by active protease. Notably, FIXa plasma clearance was relatively prolonged with ~50% of recovered antigen levels present at 25-30 min post-injection. Similar to FIX, the pattern of FIXa plasma clearance suggested a 2-compartment model with initial and terminal phases of elimination. FIXa WT demonstrated approximately ~23% recovery of predicted levels at 5 min in HA mice, similar to the zymogen (21%) in HB mice (Table I). FIXa R150A (reduced AT affinity) demonstrated markedly enhance recovery (43%) in HA mice relative to WT, while FIX R150A (24%) in HB mice which was similar to WT. FIXa K126A/132A (reduced heparin affinity) demonstrated markedly enhanced recovery (55%) in HA mice, slightly enhanced relative to the similarly increased zymogen recovery (48%) in HB mice. FIXa WT-GGACK (active site inhibited) demonstrated similar recovery to the unmodified FIXa WT with a significantly faster rate of clearance (2.2 fold) in the initial phase (k1) and a significantly slower rate of clearance (3.6-fold) in the terminal phase (k2). Overall, the initial rate (k1) was not significantly different among the active FIXa variants in HA mice or among zymogen variants in HB mice. However, comparison of individual proteases variants to their respective zymogens demonstrated that the initial rate (k1) of plasma clearance was 2-3 fold faster for the proteases, while the terminal rate (k2) was 5-9 fold faster (FIXa R150 had the smallest relative increase).
Conclusions:
The 2-phase elimination pattern for FIXa suggests that significant amounts of protease may exist in a non-circulating extravascular pool, similar to the zymogen. Further, clearance of FIXa antigen from mouse plasma is prolonged, consistent with the half-life of FIXa activity in human plasma (~41 min). FIXa variants with reduced affinity for AT (R150A) or heparin (K126A/K132A) showed enhanced recovery compared to FIXa WT, suggesting that these protease exosites make independent contributions to the extravascular binding of FIXa. The heparin exosite makes a similar contribution to zymogen binding to extravascular sites, but the AT exosite does not, likely due to being unavailable in the zymogen. Active site inhibited FIXa (GGACK) demonstrates an accelerated initial elimination phase relative to FIXa WT, suggesting re-distribution of the protein is conformation-dependent. The subsequent terminal elimination phase was slowed, consistent with an inability to be inhibited and cleared by AT
Sheehan:Pfizer: Research Funding; Bioverativ: Consultancy; Genetech: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.