Background: Advances in the understanding of the genetic determinants of AML and the widespread use of next-generation sequencing (NGS) have led to the refinement of prognostically distinct molecular subgroups. Mutations in ASXL1 and SRSF2, which are common in myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPNs), rarely co-occur in patients (pts) with AML. The largest reported cohort (n=15) of ASXL1/SRSF2 co-mutated AML had no long-term survivors (Papaemmanuil et al. NEJM 2016). It remains unknown how clinical factors such as prior history of a myeloid neoplasm or intensity of treatment influence outcomes. We sought to assess the clinical characteristics and analyze outcomes in a larger cohort of pts with ASXL1/SRSF2 co-mutated AML. We hypothesized that this profile may be a genomic footprint of prior myeloid neoplasia.
Methods: We conducted a multi-institutional retrospective analysis of newly diagnosed adult AML pts with both ASXL1 and SRSF2 mutations at the University of North Carolina and at Moffitt Cancer Center from 2011-2018. NGS was performed on DNA using the Illumina TruSight Myeloid 54-gene sequencing panel. The primary endpoint was overall survival (OS) defined as time from diagnosis of AML to death. Pts were stratified by secondary AML (s-AML), defined as having a documented history of MDS/MPN. Secondary outcomes included rates of complete remission (CR) and CR with incomplete hematologic recovery (CRi). Multivariable analysis was performed with baseline characteristics.
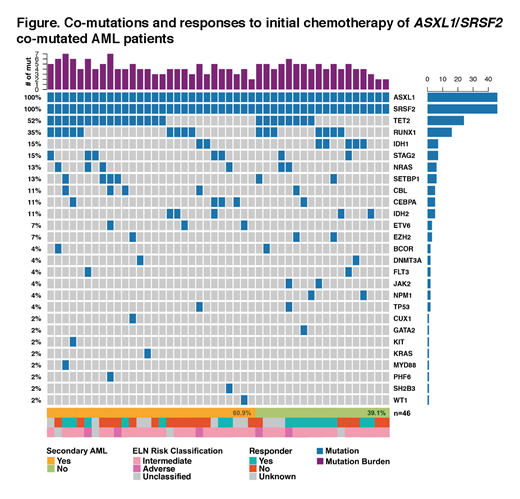
Results: Forty-six pts were identified and included. The median age of pts was 72 years (range 42 - 85). Sixty-seven percent (28/42) had normal cytogenetics; 88% (37/42) were intermediate risk cytogenetics by current ELN guidelines. Sixty-one percent (n=28) were classified as having s-AML. One pt had therapy-related AML without preexisting MDS/MPN and was therefore not included in s-AML. The Figure illustrates co-existing mutations and individual responses to upfront therapy stratified by s-AML and non-s-AML. The median number of mutations was 5 (range 2 - 7). The most common co-occurring mutations were TET2 (52%), RUNX1 (35%), IDH2 (15%), and STAG2 (15%).
Median OS was 7.0 months (m) (CI 5.3, 15.4). Median OS for pts with s-AML (n=28) and non-s-AML (n=18) was 6.1 and 15.4 m (p=0.05), respectively. There was no significant difference in median OS between s-AML and non-s-AML on multivariable analysis (hazard ratio (HR) = 2.56, p=0.07). Median OS did not differ by age (Age <65 years v. older, p=0.54), total # of mutations (≥ 5 v. less, p=0.73), or etiology of s-AML (MDS v. MPN, p=0.66). Twenty-two (47%) pts received upfront intensive induction chemotherapy (IC), 17 (37%) received hypomethylating agents (HMAs), and 7 pts (15%) received no AML-directed chemotherapy. Median OS did not significantly differ between pts receiving upfront IC and HMAs (15.3 v. 7.04 m, p=0.21). Among non-s-AML pts, median OS was longer in those receiving IC (n=10) versus HMAs (n=7) (15.4 v. 3.5 m, p=0.01). Among all pts receiving IC, median OS was longer in non-s-AML pts (n=10) versus s-AML pts (n=12) (15.4 v. 5.9 m, p=0.01). Median OS did not differ by treatment intensity for s-AML pts (IC v. HMA: 5.9 v. 9.9 m, p=0.38). Six pts underwent allogeneic hematopoietic cell transplant (HCT) with a median OS NR (median follow-up 15.6 m).
Overall rate of CR/CRi was 35% and was similar between pts receiving IC and HMAs (45% v. 21%, p=0.29). Among pts with non-s-AML, CR/CRi rates with IC and HMAs were 70% and 29%, respectively (p=0.11). Among pts with s-AML, CR/CRi rates with IC and HMAs were 42% and 20%, respectively (p=0.38).
On multivariable analysis of baseline characteristics, only ECOG performance status (PS) was significantly associated with OS (HR 2.25, p=0.01). ECOG PS remained significant (HR 2.65, p=0.03) after adjusting for HCT and treatment intensity.
Conclusions: ASXL1/SRSF2 co-mutated AML represents a rare but distinct genotype with most pts having pre-existing myeloid neoplasms and associated co-mutations commonly seen in MDS/MPNs. OS is dismal regardless of age, number of mutations, treatment intensity, or prior history of myeloid neoplasm. HCT may mitigate these poor outcomes and lead to long-term survival. This represents the largest reported cohort to date of pts with ASXL1/SRSF2 co-mutated AML. Further study is warranted to inform risk stratification and prognosis of pts with ASXL1/SRSF2-mutated AML.
Foster:Bellicum Pharmaceuticals, Inc: Research Funding; Daiichi Sankyo: Consultancy; MacroGenics: Research Funding; Celgene: Research Funding. Coombs:Octopharma: Honoraria; Pharmacyclics: Honoraria; Medscape: Honoraria; Abbvie: Consultancy; Loxo: Honoraria; Cowen & Co.: Consultancy; Dedham Group: Consultancy; H3 Biomedicine: Honoraria; Covance: Consultancy. Sallman:Celyad: Membership on an entity's Board of Directors or advisory committees. Zeidner:Agios: Honoraria; Celgene: Consultancy, Honoraria, Research Funding; Daiichi Sankyo: Honoraria; Tolero: Honoraria, Research Funding; Pfizer: Honoraria; AsystBio Laboratories: Consultancy; Merck: Research Funding; Takeda: Research Funding; AbbVie: Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.